
Nitrogen project listing 2023
Nitrogen+Syngas’s annual listing of new ammonia, urea, nitric acid and ammonium nitrate plants.

Nitrogen+Syngas’s annual listing of new ammonia, urea, nitric acid and ammonium nitrate plants.
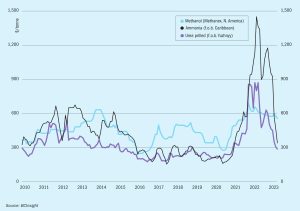
Further downward corrections are possible but the rate of demand is stabilising, suggesting the market floor is in sight, though some have suggested that May could bring another sharp reduction in the Tampa contract price towards the mid$300s c.fr. Demand remains sluggish in both eastern and western hemispheres.

In 1967 Stamicarbon revolutionised the urea process by the invention of the high-pressure CO2 stripper by Petrus J. C. Kaasenbrood. The high-pressure CO2 stripper led to following benefits:

Last year, in the wake of Russia’s invasion of Ukraine and the associated disruption to fertilizer and grain exports from both countries, there were dire predictions of the impact upon global food supply. That the worst of these predictions have not so far come to pass is in no small part due to the deal brokered by the United Nations and Turkey in July 2022 to allow exports of grain and fertilizers from Black Sea ports. According to the UN, since last July, some 29.5 million tonnes of grain and foodstuffs have been exported from Ukraine via the Black Sea, including nearly 600,000 tonnes in World Food Programme vessels for aid operations in Afghanistan Ethiopia, Kenya, Somalia and Yemen. Before the war, Ukrainian grain fed the equivalent of up to 400 million people worldwide, and the deal ensured that Ukrainian grain exports ‘only’ fell by 5 million t/a over the past year.

The inaugural Argus Sustainable Fertilizer Americas Conference is being held at the Grand Hyatt Tampa Bay, Tampa, Florida, USA, 5-6 June 2023.

More than 370 delegates from over 160 companies and 40 countries gathered at the Hilton Bomonti Hotel, Istanbul, Turkey, 27 February to 1 March, for CRU’s Phosphates 2023 conference.

Anglo American’s CEO Duncan Wanblad spoke in detail about the company’s new strategy for the Woodsmith mine during an annual results presentation on 23rd February. Selected highlights are provided below.

Alzbeta Klein, CEO and Director General of the International Fertilizer Association (IFA), sets the scene for IFA’s Annual Conference in Prague, 22-24 May.

The Sustainable Fertilizer Academy (SFA) offers everyone within the fertilizer industry access to education on sustainability via a certified e-learning programme. More than 150 students have registered to date and over 40 alumni have graduated from the academy since it opened in September last year.

Anglo American recently unveiled the long-awaited strategy update for its large-scale Woodsmith mine project in the UK. Initial production of the company’s POLY4 polyhalite fertilizer is now scheduled to begin in 2027. The mine’s ultimate annual output has also been increased to 13 million tonnes.