Nitrogen+Syngas

30 June 2016
Problem No. 36: Change in colour of urea product
Stainless steel owes its corrosion resistance to the presence of a protective chromium oxide layer on the surface. As long as this layer remains intact, the metal corrodes at a very low rate. If the passive layer in stainless steel is damaged, active corrosion starts in aggressive corrosive environments. Stainless steels exposed to carbamate containing solutions in the urea synthesis section can be kept in a passive state by adding a minimum amount of oxygen. If the oxygen content drops below this minimum, active corrosion starts, which can be identified by a shiny silver colour on the surface (see picture). Adding oxygen and maintaining a sufficiently high oxygen content in the various process streams are prerequisites for preventing excessive corrosion of the equipment and piping. Once active corrosion occurs, the corrosion rate is extremely high (>50 mm/ year) and will not stop even if more oxygen is supplied to the process.
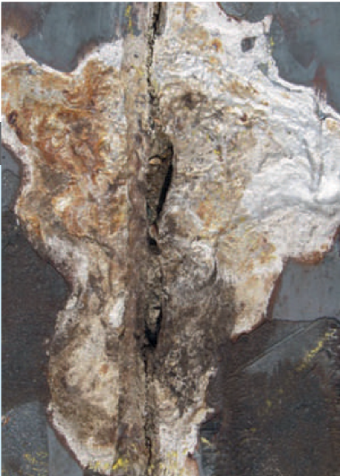
Left: Corrosion of an internal part. Right: Corrosion of a valve gland.
Mr Akbar Ali from SAFCO, Saudi Arabia starts off the discussion:
What are the possible causes for a change in urea product colour e.g. a dull white colour?
Mr Mark Brouwer from Ureaknowhow.com, The Netherlands, provides the first answer: I know of two causes: oil and corrosion products (iron oxides). Check the nickel content in the prills and compare it to the normal level and/or check for high oil consumption somewhere. A low level in a tank could indicate that oil is passing to the downstream sections.
Mr Nasir Hussain from Pak Arab Fertilizers Multan in Pakistan joins the discussion and provides further information based on his experiences: Oil and corrosion products are the most common causes of a change in prill colour. Another reason can be if the urea solution is held for a longer time in the urea melt storage tank. When we recycle this urea with fresh urea melt it results in a colour change. To check for corrosion products samples can be collected at the exit of the reactor, stripper, medium pressure decomposer, low pressure decomposer etc. to determine the exact location.
Mr Faisal Ghafoor from Fertil, United Arab Emirates shares his experience: The presence of methanol in the CO2 from the ammonia plant can also causes a colour change; this can be observed with a new LTS catalyst.
Mr Sam QR from Fertco company in India confirms Mark’s recommendation: As Mark said, it is mostly the corrosion product you will see after a stoppage or load reduction. Check for iron and nickel in the product.
Mr Malik Sohail from SAFCO in Saudi Arabia shares his experience: Yes the possible causes of product colour change have been provided by Mark. Of these I believe that during plant operation the causes are a high oil content from the pumps and compressor and/or an abrupt drop in the recovery level and concentration sections. This can be indicated by the level glass of the final separator. Foaming in the first stage evaporator can be seen form the level glass. When ammonia is imported from ammonia storage it is possible for it to have a high oil content.
Mr Muhammad Farooq from SAFCO in Saudi Arabia asks Akbar a question: Is this a permanent change of colour of the urea product or did you observe it during foaming or excess ammonia at the concentrator and rectifying column?
Akbar summarises and provides more information on the issue: Thank you for your inputs. We experienced this problem during a start-up after holdup of urea solution in the reactor for 48 hours. Just before shutdown we encountered a problem of increased H2 slippage up to 1.4% (for 15 days) with CO2 from the ammonia side; due to a problem in the ammonia plant, the delta temperature across the hydrogen reactor approached 68°C.
We suspect that during this time it was not possible to manage the air flow control properly, as seen from the oxygen content trend downstream of the hydrogen reactor. This would have resulted in active corrosion which probably caused the change in product colour. After three days of operation the original colour was resumed.
Mr Majid Mohammadian from PIDMCO in Iran shares his opinion: Contamination with impurities from the recycle tank or urea formaldehyde tank (in case of use) could be another cause.
Mr Muhammad Kashif Naseem from SABIC in Saudi Arabia gives his recommendations: From my experience, product colour can change due to the following:
- high oil contents in produc;
- high iron contents;
- carry-over of CO2 system absorbent to the synthesis loop.
Please confirm the passivated air range of between 0.6-0.8 vol-% in CO2, which is dependent upon the N/C ratio and the material of construction of the HP synthesis system. During a colour change measure the oil, Fe and Ni content in the product at different sample points. During the hold-up time whether in the reactor or the urea solution tank, the product colour always changes during start-up.
Mr Victor Rengel from FertiNitro in Venezuela asks a new question: I would like to take advantage of this discussion to ask about the normal limits for the nickel content in urea during reliable plant operation. In the last few days we had a plant shutdown with a delay of three days for recuperation. Yesterday after start-up we noted a yellow colour in the urea granules. We checked the nickel content, which was 4.44 ppm, much higher than the normal average of 0.02 ppm. We have stopped feeding liquid urea from the tank. We would like your opinion or advices about the high Nickel from the point of view of damage to the passivation layer or equipment corrosion.
Mark replies: In the case that your passive layer is not fully intact and as a result there is active corrosion, the active corrosion will continue even if you increase the oxygen content.
If you have active corrosion somewhere, you will see an increasing trend of your nickel level. There is no normal limit, every plant has its typical normal nickel level depending on materials used etc.
Victor provides further information: After stopping the feed from the urea liquid tank the nickel level is decreasing back to its normal value, therefore is no active corrosion.
Mr Nimesh Maurya from KRIBHCO in India shares his opinion: As mentioned earlier, the colour change of the urea prills is due to two main reasons: corrosion and oil contamination.
When oil seals of the ammonia compressor or carbon dioxide compressor leak oil into the system, it can be diagnosed by viewing the glass window of the MP urea holder and LP holder. If there is any foaming it means oil is coming into the system.
If oil is entering with liquid ammonia it can be removed by draining the ammonia receiver tank for some time. When you drain it, lumps of oil will come out. The ammonia receiver tank needs to be drained regularly until the leakage problem is solved.
Akbar comes back with more information: The intent of this post is to share our problem related to blocking In and its impact on product quality at one of unit (1,800 t/d Stamicarbon technology with fluid bed granulation). We are facing a frequent problem regarding off specs product due to its dull white colour, this is a common thing now whenever urea solution is kept under blocking in for 24 hours or even less than 24 Hrs.
During a recent shut down (April 29, 2014), urea solution was kept for about 25 hous for blocking in. After start up, for more than two days product was off spec due to a dull white colour. Regular analysis for Fe and Ni in the final product was carried out and it was found that the Fe content was very high (up to 5 ppm compared to the normal figure of <0.1 ppm) ), but in all samples the Ni content was < 0.1 nppm which is acceptable and normal for us.
We checked the synthesis parameters before and during blocking in as below:
- O2 to stripper as per analyser was 0.66 vol-%:
- reactor temperature during blocking in came down to 140°C;
- synthesis pressure came down to 54 kg/cm2;
- N/C was operating 2.98 to 3.0;
- CO2 feed was cut first as the CO2 compressor was tripped, NH3 feed was cut after 3 minutes;
- When product becomes normal, Fe in final product was analysed and found to be normal <0.1 ppm;
- the material of the reactor liner, down comer and trays is 316L UG (BC.01).
We are investigating this incident and seeking technical assistance concerning this problem.






