Nitrogen+Syngas 399 Jan-Feb 2026
19 January 2026
Membrane uses in methanol production
DECARBONISATION
Membrane uses in methanol production
Global demand for methanol is set to increase fivefold by 2050, driven by decarbonisation in shipping and other hard-to-abate sectors. Geir Arne Johansen and Hallgeir Angel of Air Products Membrane Solutions discuss how membrane technology offers a proven, scalable solution to improve efficiency, reduce emissions, and enable compliance across both traditional and low-carbon methanol pathways.
Low-carbon methanol market
Global methanol demand is projected to grow from ~100 million tons today to over 500 million tons by 2050, with e-methanol expected to account for up to 250 million tons. Shipping is the primary driver of e-methanol adoption. E-methanol is methanol produced by combining green hydrogen, generated from renewable electricity via electrolysis, with captured CO2 from sustainable sources. China, the Nordics, the US, Spain and Germany are emerging as global leaders in both the production and adoption of e-methanol, with commercial-scale flagship projects active and a growing pipeline of industrial offtake agreements signalling strong regional demand.
As of 2025, the EU’s Fuel EU Maritime regulation is in force, requiring ships over 5,000 gross tonnage (GT) to reduce fuel greenhouse gas intensity by 2% compared to 2020 levels. Non-compliance triggers a fixed penalty of €2,400/t of fossil fuel equivalent, which translates to roughly €39/t in 2025 and will rise to ~€353/t by 2035. Shipping companies are already adjusting operations, with carriers like Maersk adding surcharges of €60–75 per container to cover compliance costs. The first commercial-scale penalties will be settled in mid-2026, based on 2025 fuel usage and verified emissions.
E-methanol currently costs two to four times more than conventional methanol, but carbon pricing and incentives are narrowing the gap. Economies of scale and policy support are expected to make e-methanol cost-competitive, positioning it as a cornerstone fuel for transport and industry Bio-methanol currently costs more than fossil methanol, but incentives for carbon removal and circular feedstock are improving its competitiveness. Bio-methanol is methanol made from biomass feedstocks, offering a renewable and potentially carbon neutral alternative to fossil methanol. As biomass supply chains scale and carbon credit markets mature, bio-methanol is expected to become a cost-effective solution, positioning it as a critical component of the low-carbon fuel mix for shipping and industry.
Future trends and applications
In 2025, e-methanol reached a significant milestone with the launch of the first commercial-scale plants in Denmark and China, marking its transition from pilot projects to industrial production. Demand has surged across hard-to-abate sectors like shipping and chemicals, driven by regulatory pressure and corporate climate commitments.
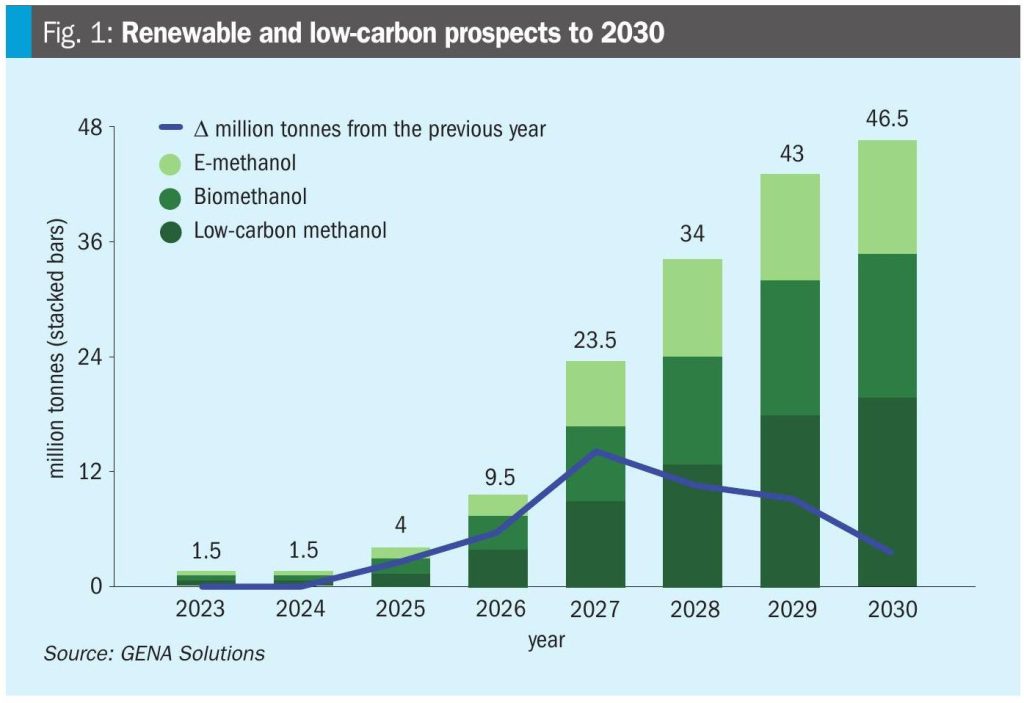
Future trends point to rapid global expansion, with over 20 large-scale projects underway and a projected renewable methanol capacity of up to 14 million tons by 2030. The portion of projects expected to be operational by 2028 could reach 34.2 million tons, up from just 3.8 million tons in 2025 (Fig. 1), reflecting a compound annual growth rate (CAGR) of over 100% between 2025 and 2028.
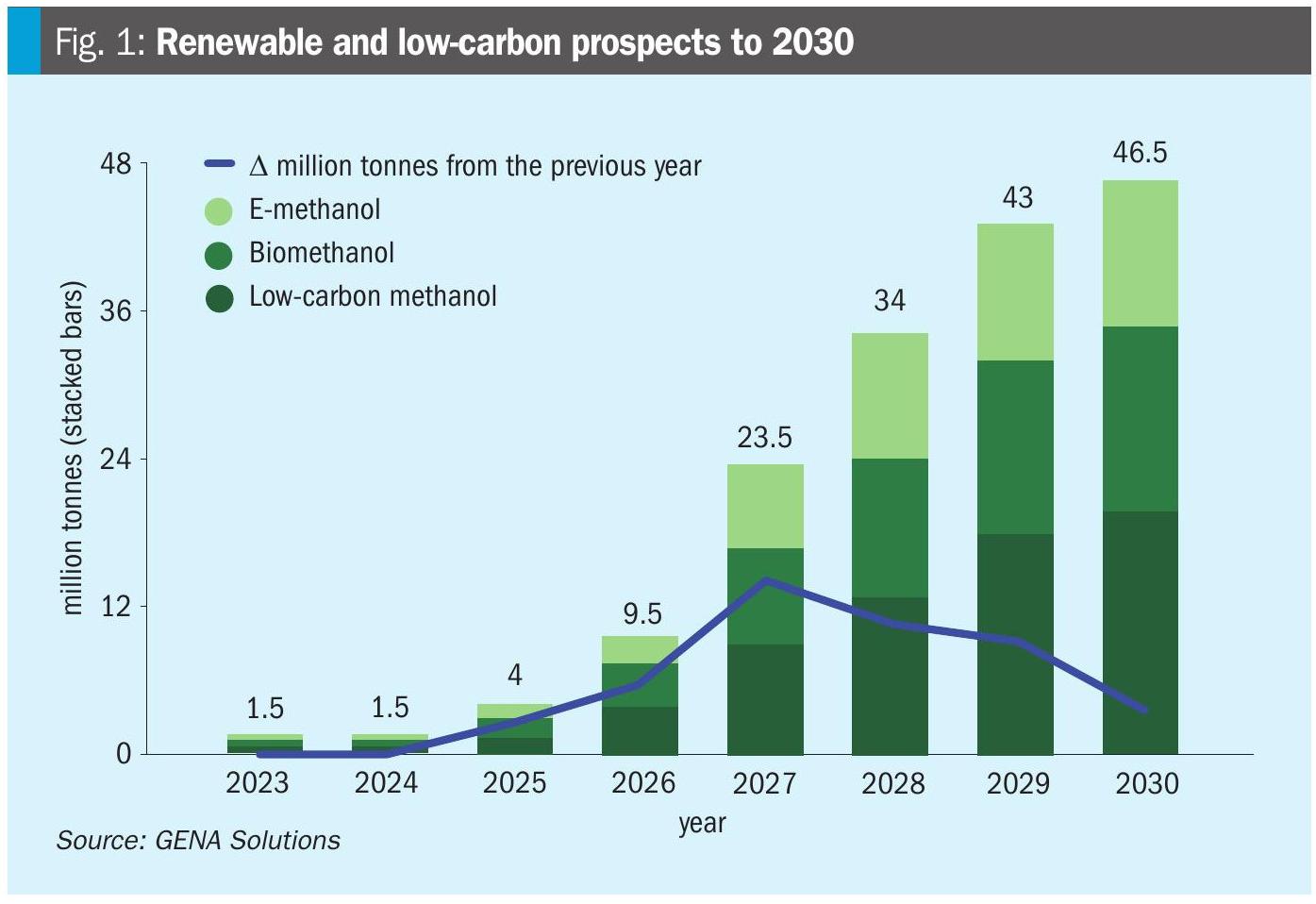
Policy incentives such as the US Inflation Reduction Act and EU’s Fit for 55 package are accelerating adoption by subsidising green hydrogen inputs and penalising fossil fuel.
E-methanol also shows promise in aviation as a precursor for sustainable aviation fuels (SAF), particularly through methanol-to-jet (MtJ), a specific alcohol-to-jet (AtJ) pathway, and power-to-liquid (PtL) routes (Fig. 2) that can deliver near-zero lifecycle emissions. Compared to other bio-based SAF options such as hydroprocessed esters and fatty acids (HEFA) and AtJ pathways using ethanol or other alcohols, e-methanol derived jet fuel offers superior carbon reduction potential, up to 99% lower CO2 emissions, while also avoiding land use and feedstock constraints. Although current production costs remain higher than fossil jet fuel, policy incentives such as the US Inflation Reduction Act and EU ReFuelEU mandates are actively narrowing the gap and guaranteeing market demand.

As renewable electricity and carbon capture technologies scale, e-methanol is increasingly viewed as a cornerstone molecule for aviation decarbonisation, with long-term potential to dominate SAF supply. Its versatility, scalability, and alignment with global net-zero targets position it as a strategic enabler of the low-carbon energy transition Further e-methanol is emerging as a compliance-ready marine fuel under stringent climate regulations such as FuelEU Maritime and IMO net-zero targets. It enables shipping operators to meet GHG intensity reduction mandates while avoiding escalating penalties and reputational risk. With growing offtake agreements, fleet conversions, and policy support, e-methanol is positioned as a cornerstone solution for maritime decarbonisation.
Low-carbon methanol pathways
Methanol can be produced through various routes (Fig. 3), ranging from traditional coal and natural gas feedstock-based methanol (brown and grey) to biomass and renewable-based methanol (blue and green). Each pathway offers different benefits in terms of carbon footprint, feedstock flexibility, and regulatory compliance. Fig. 3 shows where PRISM® membranes have been or could be utilised to improve the efficiency of each process step.
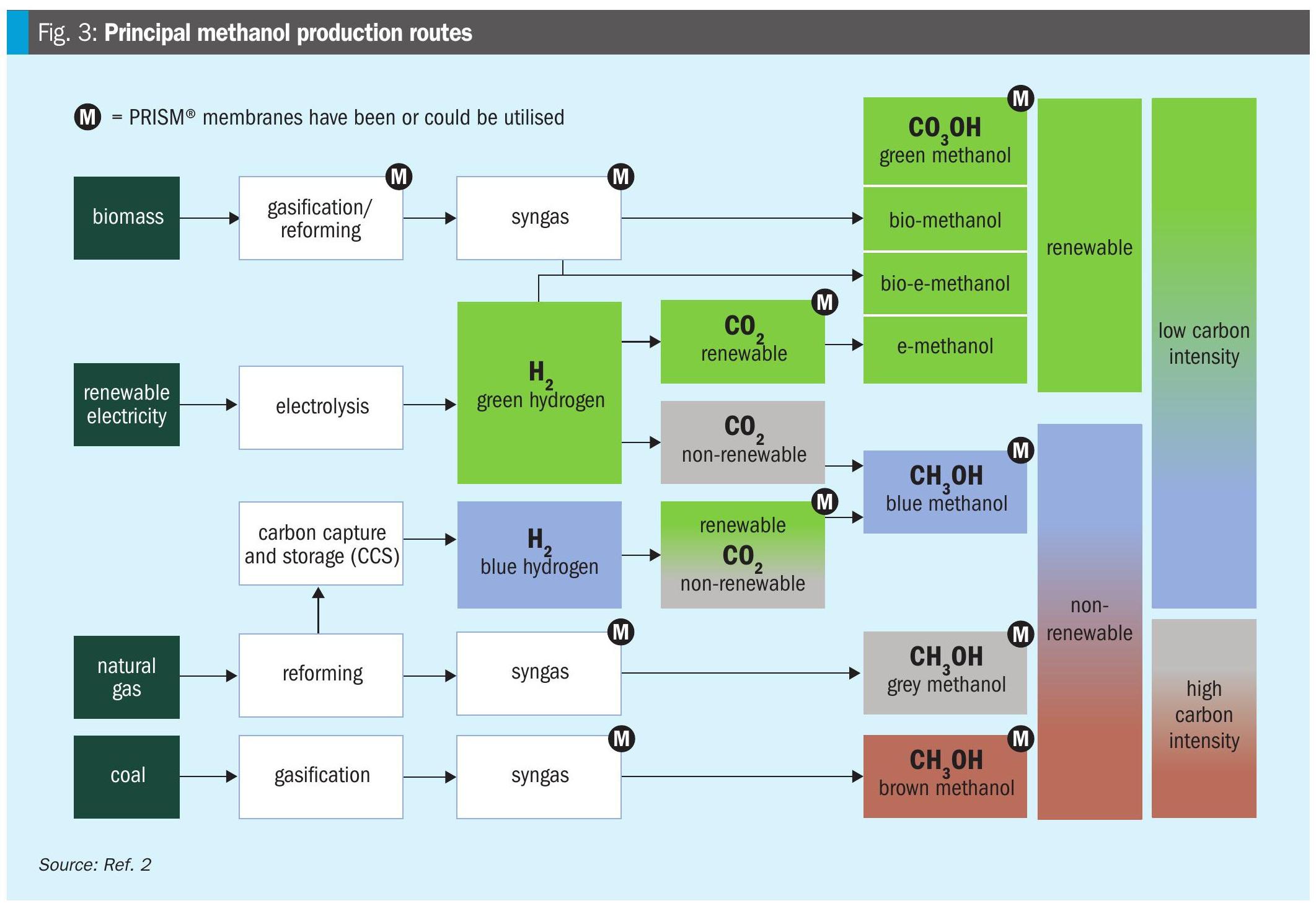
The global shift toward low-carbon methanol is evolving into full-scale industrial production, driven by regulatory pressure, commercial demand, and technological maturity. Four distinct pathways define this transformation:
Carbon capture-integrated reforming
Blue methanol facilities integrate carbon capture into fossil-fuel reforming and/or the methanol synthesis loop, intercepting CO2 emissions pre- or post-combustion. Captured CO2 is stored in geological formations or used for enhanced oil recovery, reducing lifecycle emissions by more than half compared to conventional methanol. This pathway is attractive for regions with abundant gas and infrastructure but is reliant on permanent CO2 storage integrity.
Biomass gasification with CCS
Biomass can be converted to syngas by gasification, or alternatively by reforming of biomethane produced by anaerobic digestion of biomass. These pathways can capture biogenic CO2, achieving net-negative emissions. It actively removes carbon from the atmosphere, making it compelling for shipping and aviation. Scalability depends on feedstock availability, logistics, and land-use considerations.
Electrochemical CO2 conversion
Electrochemical conversion uses electricity to directly reduce CO2 into methanol within an electrochemical cell, bypassing separate hydrogen production and high-temperature synthesis. Modular and low temperature, it enables distributed methanol production at industrial sites and capture and conversion of flue gas on-site.
When powered by renewable electricity, this pathway offers near-zero carbon methanol with minimal infrastructure.
Hybrid cycles
Hybrid cycles combine renewable hydrogen with captured CO2 from flue gas, biomass combustion, or direct air capture. Designed for retrofits and new builds, these systems adapt to local carbon and energy conditions. Recognised under EU RED III and the US Inflation Reduction Act, these cycles deliver 70–90% lower emissions than fossil routes.
Membrane roles in methanol production
Fundamentals of membrane separation
PRISM® membrane separators use the principle of selective permeation of gases through semi-permeable barriers. Molecules of a gas will move through or “permeate” a membrane barrier if the partial pressure of the particular gas is lower on the other side (Fig. 4).
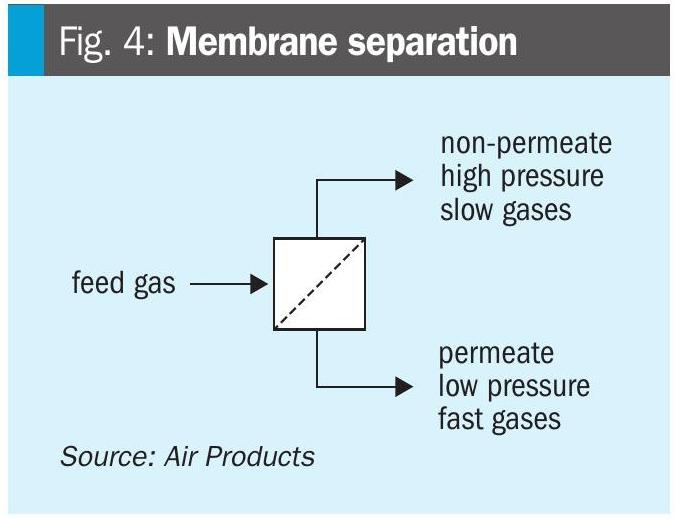
Different gases permeate at different rates, independent of their partial pressures in the process stream. It is the differences in these permeation rates by which membranes affect gas separation.
Hydrogen is a fast-permeating gas (Fig. 5) and can efficiently be separated from other slow-permeating gases.
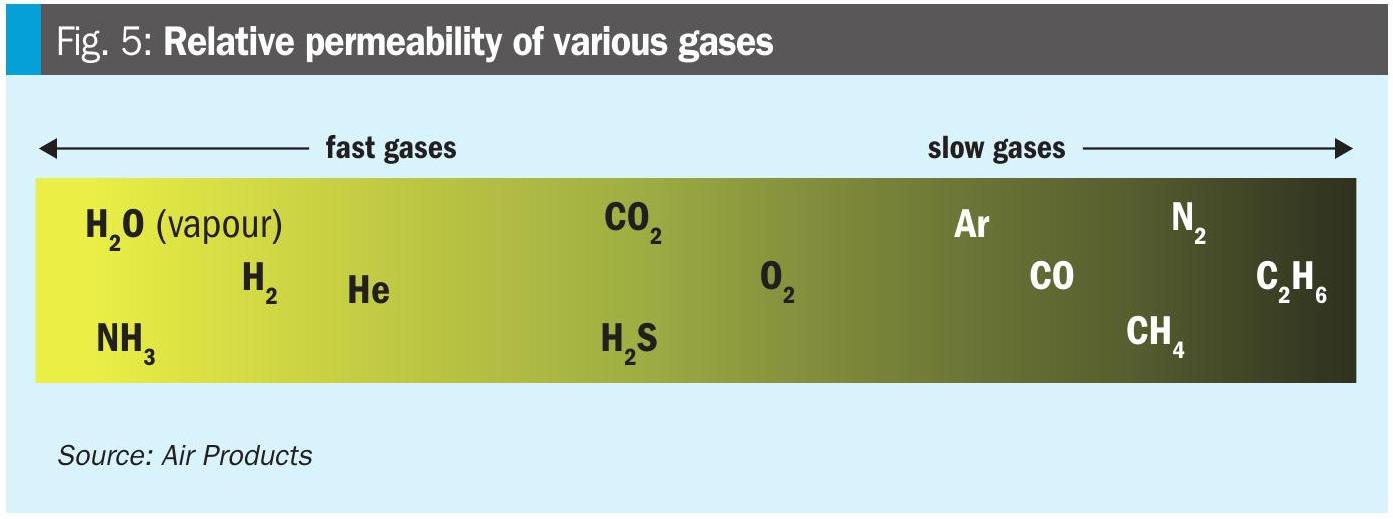
Applications
H2 recovery of synthesis loop purge gas for brown and grey methanol
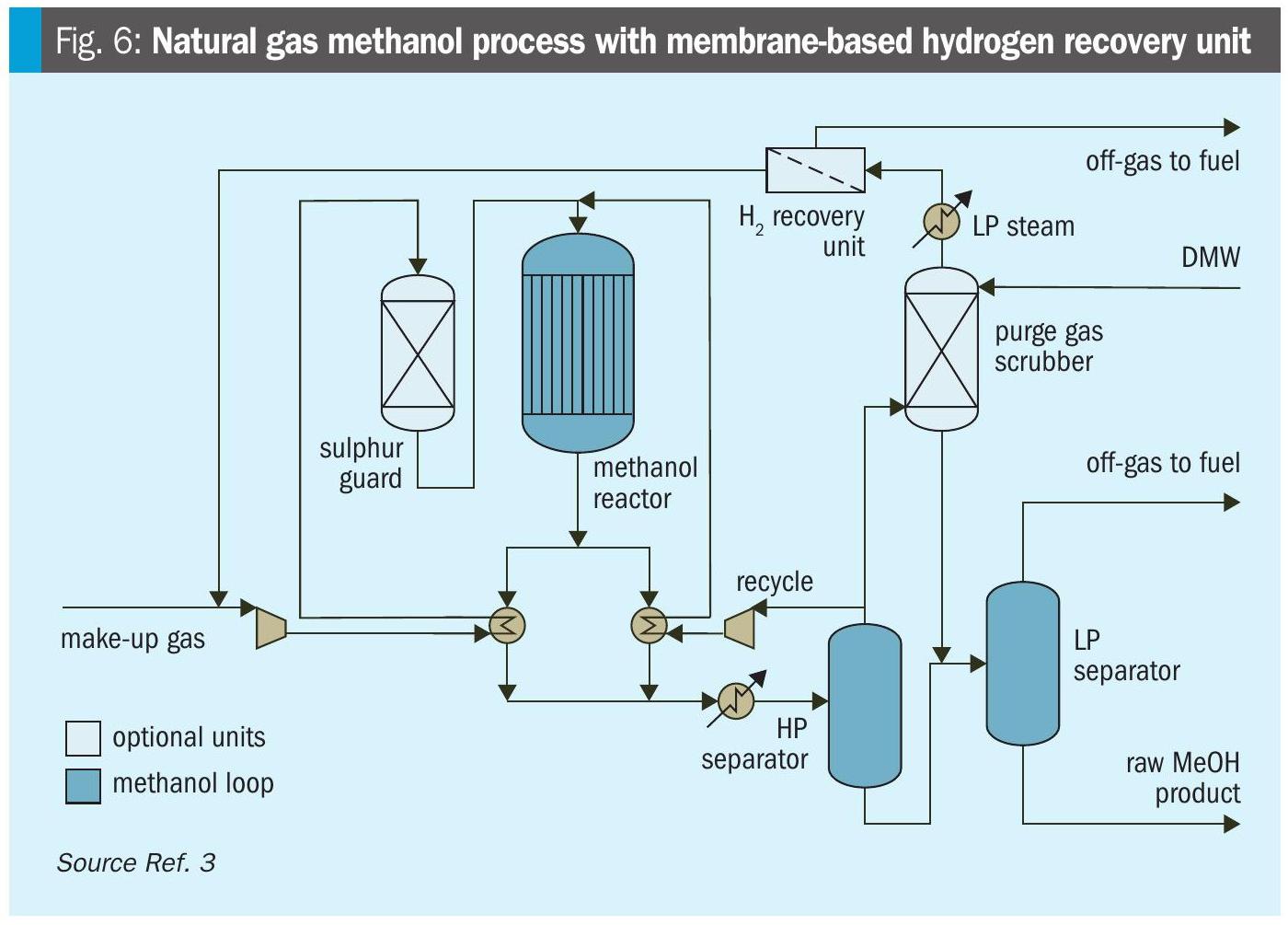
Air Products PRISM® membranes were successfully developed and implemented for hydrogen separation of process gases in the late 1970s, for which the technology received the 1981 Kirkpatrick Chemical Engineering Achievement Award. Since the first commercialised, large-scale PRISM® membrane unit for methanol purge was built in 1982, Air Products has delivered more than 80 systems for methanol purge gas applications. Installed at both coal gasification and natural gas-based methanol plants (Fig. 3), creating great value and reduced CO2 emissions for customers.
A PRISM® membrane purge gas recovery unit improves methanol plant efficiency by integrating into the synthesis loop (Fig. 6). In the process, a portion of the synthesis gas is purged to prevent the accumulation of inert components such as argon, methane, and nitrogen. This purge gas also contains valuable hydrogen and carbon monoxide. The gas is heated and fed into membrane modules at high pressure, where hydrogen permeates through the membrane and is returned to the synthesis compressor suction at a lower pressure. The remaining gases, including argon, methane, nitrogen, and carbon monoxide, stay on the non-permeate side and can be used as fuel in the reforming section. These systems recover typically 85-95% of the hydrogen from the purge gas, significantly increasing methanol production without increasing natural gas feed to the reformer.
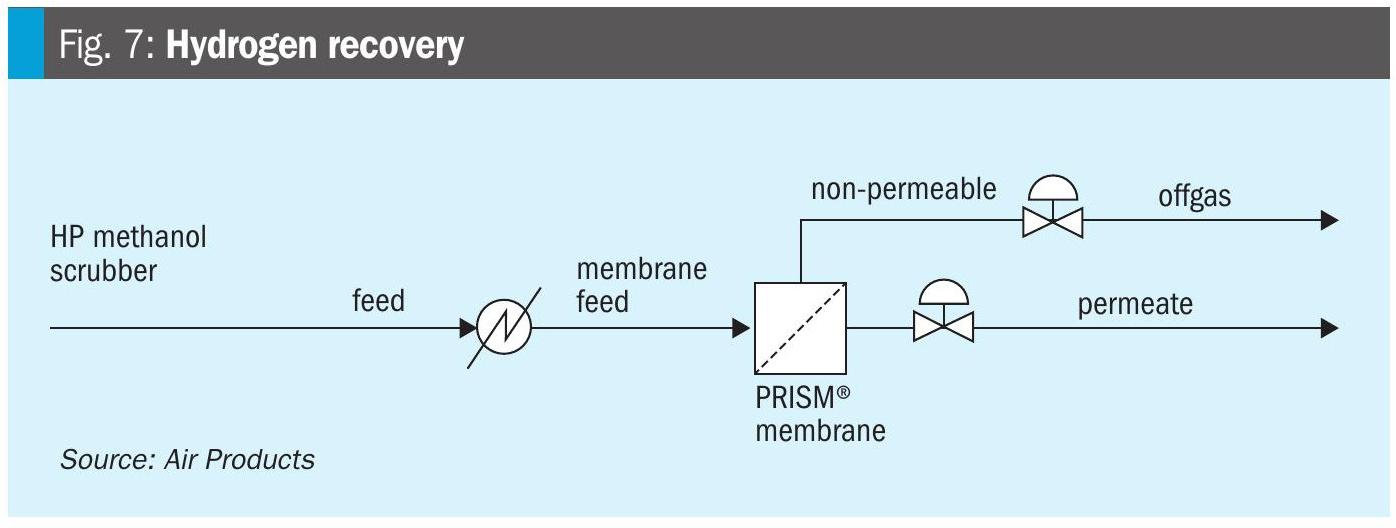
Syngas ratio adjustment with the PRISM® membrane
The syngas feed to the methanol reactor must maintain an H2/CO ratio between 2.0 and 2.5 for optimal synthesis. Syngas for methanol production can be produced from a variety of feedstocks and processes, including reforming fossil fuels or biomethane and gasification of biomass. Depending on the feedstock and reforming process, adjustment of the H2/CO ratio may be necessary.
PRISM® membranes are ideally suited for this adjustment because hydrogen, being a fast-permeating molecule, passes through the hollow fibre membrane more readily than carbon monoxide. This enables precise control of the syngas composition without complex cryogenic or chemical processes.
With over 40 years of experience and 70+ references in H2/CO ratio and CO purification applications, membrane technology is proven and reliable across a wide range of capacities from 40 Nm³/hr to over 400,000 Nm³/hr. Hollow fibre membranes provide a compact, modular solution that can be scaled easily, offering low pressure drop, high surface area, and robust performance under varying operating conditions. These systems are designed with no moving parts, thus offering unrivalled reliability and very low maintenance requirements compared to other technological options.
H2 recovery of synthesis loop purge gas for blue and green methanol
In low-carbon methanol plants, whether based on biomass or other renewable feedstocks the synthesis loop often requires a purge to prevent inert gas buildup. These purge streams contain valuable hydrogen that can be recovered using PRISM® membranes.
The same proven technology applied in traditional methanol plants can be adapted for blue and green methanol facilities, thanks to the flexibility of membrane systems in handling different gas compositions and flow rates. This recovery step improves overall plant efficiency and reduces the need for additional hydrogen production, supporting both economic and environmental goals.
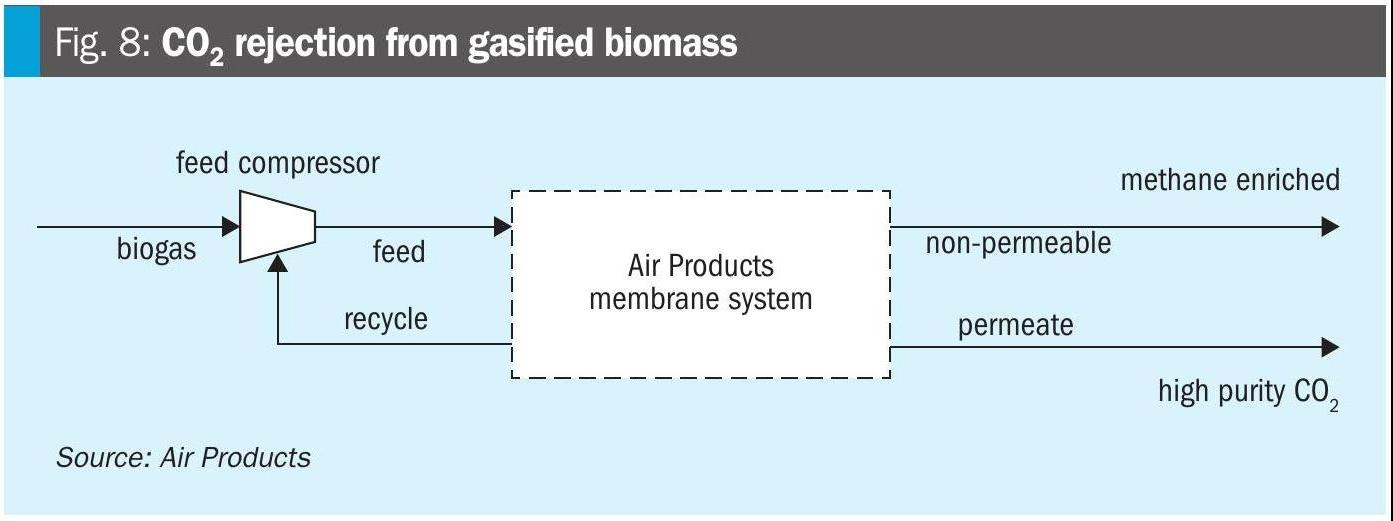
CO2 rejection from biomethane
With hundreds of references in the biogas market, PRISM® membranes are field proven and ideally suited for this application. Biomethane produced by anaerobic digestion, wastewater treatment, or landfills can be used as a feedstock for methanol synthesis. CO2 that is co-produced by these methods can be fed to the methanol synthesis plant at a predetermined ratio or recovered for carbon capture. PRISM® membranes can selectively reject CO2 while enriching methane in the non-permeate stream. PRISM® membranes simplify downstream processing by reducing CO2 load before methanol synthesis, minimising compression requirements and improving catalyst performance.
By integrating membrane-based CO2 removal with methane recovery, producers can achieve a cleaner syngas composition, enable higher methanol yields and support net-negative emission pathways when combined with carbon capture and storage.
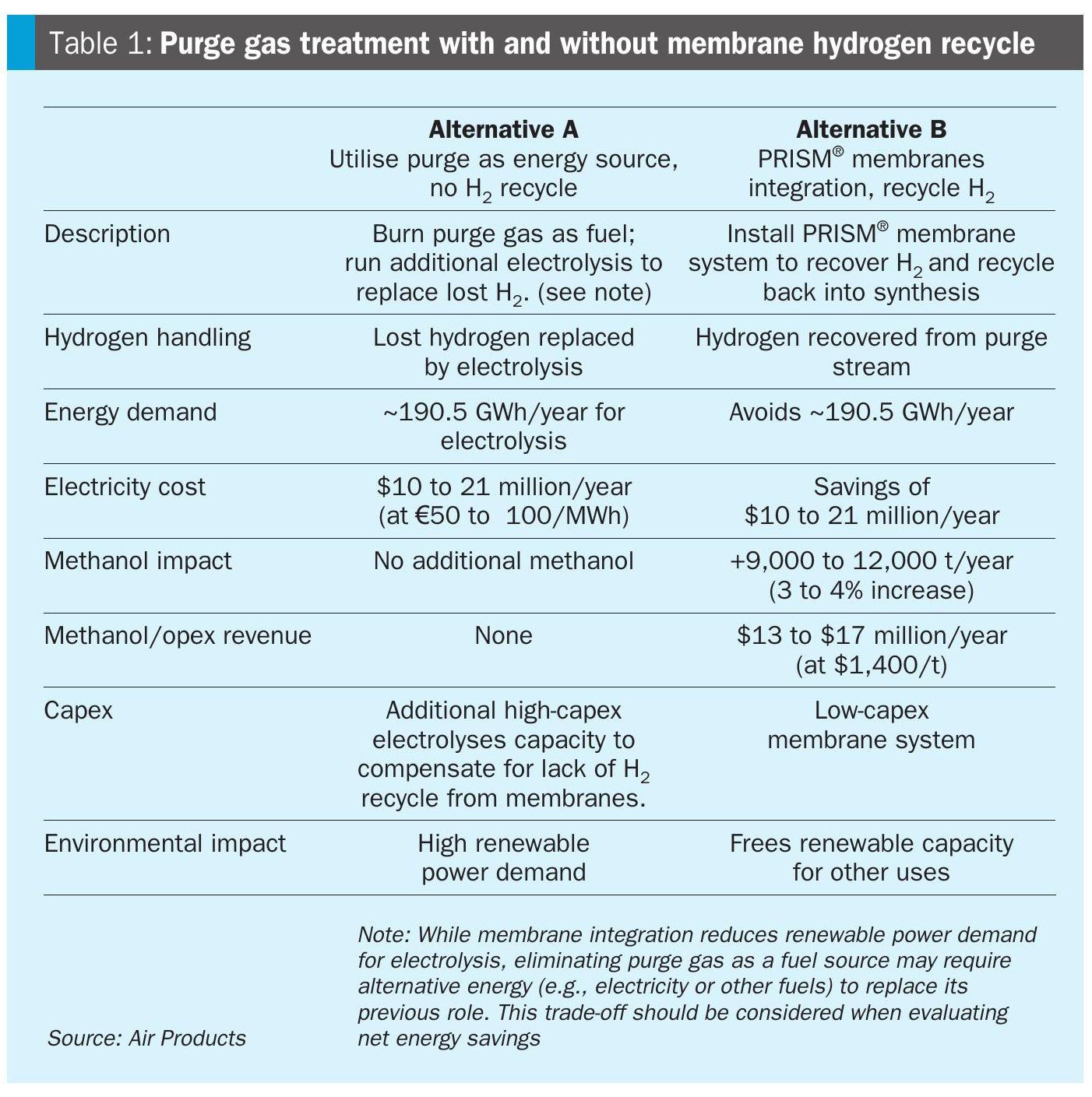
Economic impact of hydrogen recovery: Two value pathways
In low-carbon methanol plants, the synthesis loop purge contains valuable hydrogen. How this hydrogen is managed determines both the energy footprint and the economics of the plant. There are two fundamentally different approaches as identified by Alternative A and B in Table 1.
Conclusion
Membrane systems offer a low-capex, high-impact upgrade that enhances sustainability and profitability, making them a strategic enabler for next-generation methanol plants.
For a 300,000 tonne/annum green methanol plant, membranes can cut renewable power demand by ~190.5 GWh/year, free up capacity for other uses, and deliver strong economic returns, potentially $10 to 21 million/year in reduced electricity costs.
At the same time hydrogen recycled by the PRISM® membrane system adds up to $9 to $12 million/year in increased methanol production.
References





