Nitrogen+Syngas 327 Jan-Feb 2014

28 February 2014
Problem No. 22: How to strengthen prilled urea product
Approximately 75% of all urea plants worldwide still produce prills. Compared to granules, prills are smaller in size and have lower strength, leading to more dust problems during handling. Furthermore, prill strength is reduced, particularly during hot and humid weather conditions and when plant loads are increased. This Round Table discussion reports on the experiences of different plants and the solutions available to increase the strength of urea prills.
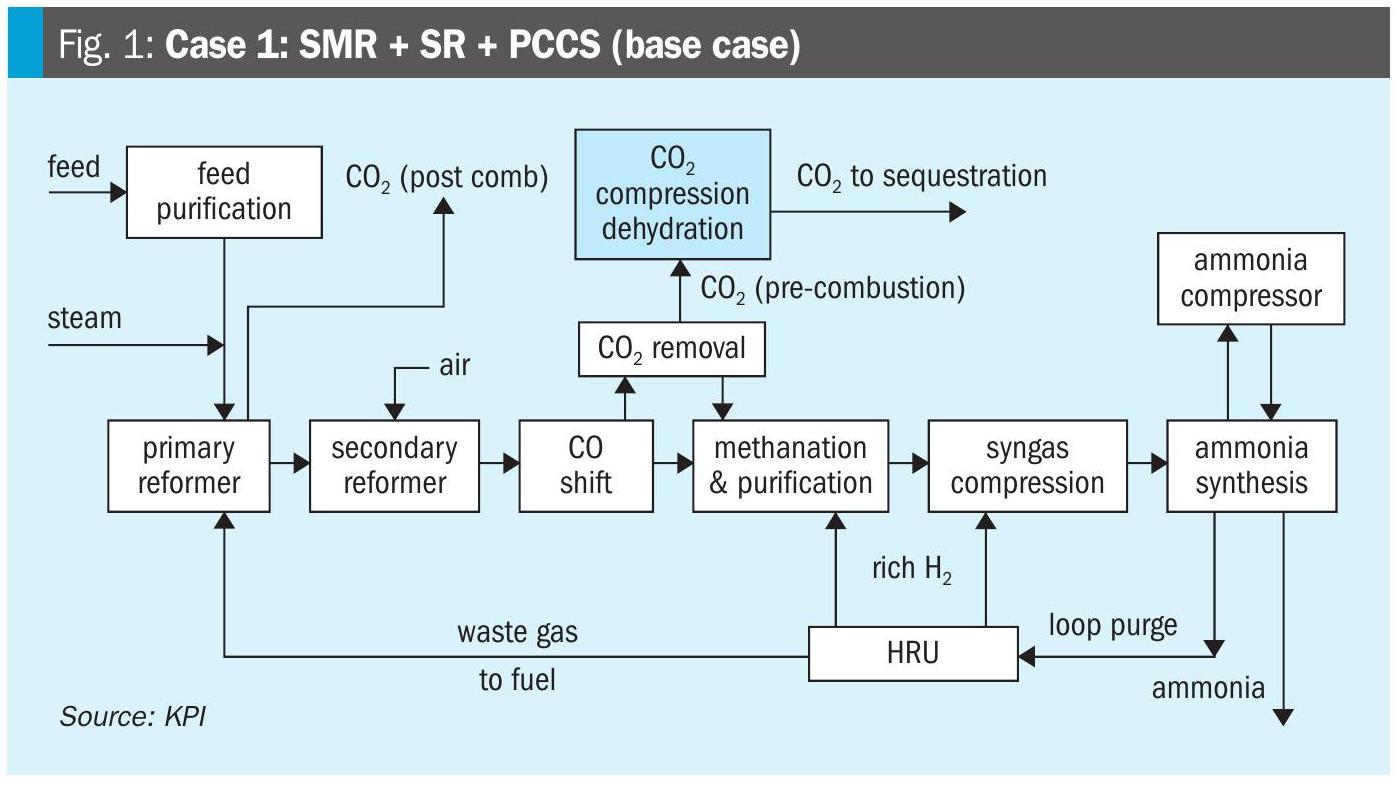
High speed record of impact of a 2 mm urea prill at impact velocity of 10m/s. Framing speed: 100,000 frames per second.

Mr Muhammad Kashif Naseem of SABIC in Saudi Arabia initiates the following discussion: We have prilled urea and its strength is very weak. We often have too much undersize. What are the causes of weak prill strength and how can it be improved?
Mr Le Ngoc Ban of Phu My Fertilizer Plant in Vietnam confirms the problem: We have the same problem. Dust in our prilled product (size < 1 mm) sometimes exceeds 1% even when we reduce the prilling bucket speed to nearly overflow speed.
Mr Salman Islam of DHCL in Pakistan provides an answer and asks a further question: One reason for excess fines could be improper cooling of urea prills and high bucket speed. Which type of prill tower do you have in your plant?
Kashif replies: Our plant has an induced draft prill tower.
Le also replies: Our plant is using a natural draft prilling tower and a tuttle prilling bucket.
Mr. Les Farbotko of Incitec Pivot Limited in Australia shares his experiences: I am not sure if your problem is broken prills and dust, or tiny prills. We experienced both of these problems several years ago, and spent a lot of time and effort trying to improve our prill quality. To make harder prills (i.e. prill which do not break as easily), we found the main factors that made a big difference to us were:
- Moisture content: Prills made with 0.3 wt-% H2O or less were noticeably harder than prills with 0.5 wt-% H2O.
- Formaldehyde: Adding 0.3 wt-% formaldehyde to the urea melt upstream of prilling makes stronger prills. (It is preferable to use urea-formaldehyde concentrates rather than aqueous formaldehyde solutions, for safety reasons)
I am reliably told that prill seeding also makes prills harder, although I can’t say how much difference it makes. To make prills with a more uniform size distribution (fewer tiny prills, and less oversize prills which form eggshells and break into dust easily), you need to pay close attention to your prilling buckets. In aluminium buckets, the holes gradually corrode or erode, becoming larger over time and requiring periodic replacement. This seems to be worse if your prilling temperature is high. We also discovered that it is not easy to drill thousands of uniformly sized holes in a prilling bucket. There is a degree of variability in hole diameter that is related to the amount of care taken, the sharpness of the drill bits and the drilling technique.
Mr Mark Brouwer of UreaKnowHow.com in the Netherlands contributes to the discussion: As regards strength of prills we need to distinguish:
- Crushing strength (how much weight a prill can withstand before it crushes)
- Impact strength (with which speed a prill can hit a wall before it is destroyed)
- Adding formaldehyde has some positive effect on the crushing strength (+10-20%)
- Seeding technology improves the impact strength of prills as it ensures that the prills consist of many crystallites.
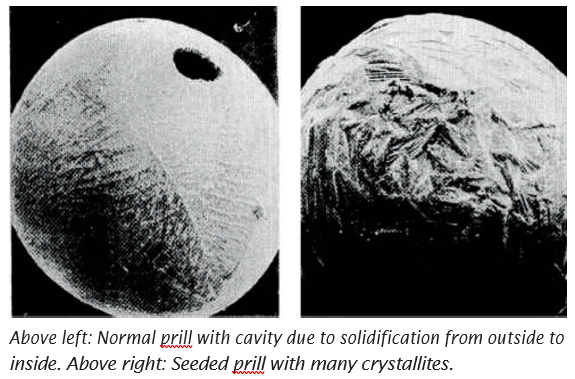
Therefore, if your prills are subjected to a lot of handling, or if the prills have to drop from high elevations during handling, seeding technology is beneficial. It should also help to reduce fines at the bottom of the prill tower. Finally, adding sulphur or sulphates also increase the crushing strength.
Mr Janusz Ma kowski of ZCh Police in Poland shares his experiences: Regarding the buckets, I agree that tuttle buckets are very sensitive and if you use them you have to check the condition of the buckets:
- Check that the geometry of the bucket doesn’t change over a long time in operation.
- Check if holes of the bucket have still the same diameter.
- Check if the holes are still sharp (not rounded after long time of operation)
If the bucket is not perfectly round, or if the diameters of the holes vary a lot, you will produce an excessive amount of dust. Check also that the bucket isn’t tarnished by impurities e.g. corrosion products. In this case the bucket should be replaced for cleaning. Adding 0.3 wt-% formaldehyde to the urea melt is a very good idea. Have you ever used a vibropriller (vibrobuckets)? What is your biuret content in final product?
Mr Son Nguyen of PVFCCO in Vietnam shares his experiences: We have a Snamprogetti plant with a natural draft prilling tower. Our product has a moisture content of: 0.27-0.3 wt-%, prill temperature of 50-55°C, biuret content of less than 1 wt-%, urea melt temperature to prilling bucket of 136-137°C and we use a Tuttle aluminum prilling bucket.
We always keep process data as close as possible to the designed values by Snamprogetti but we have found that the dust level in our product is still high: about 1% product can go through a 1 mm screen during thelLab test (screen amplitude = 65), then we tried to improve our prill strength.
While inspecting our product at the bottom of prilling tower, we detected some ammonia smell but unfortunately our lab isn’t able to measure the NH3 content in the urea melt to the prilling bucket. We thought that the free NH3 in the urea may cause our prills to be weaker by creating capillaries during the crystallization of the urea prill and by getting rid of it we could make our product stronger.
In our plant, we have a H2SO4 injecting system with the initial purpose to reduce the NH3 content in the off gas of the prilling tower and we thought it could help in getting rid of free NH3. We tried injecting some H2SO4 into the urea melt going to prilling tower, varying the amount of H2SO4 at several levels. Then we took samples to measure product strength and we got the following results:
- NH3 smell at the bottom of the prilling tower is reduced.
- Dust at the bottom of the prilling tower is reduced.
- Specific crushing strength of all samples with H2SO4 reduced than without (2 mm size).
- Common crushing strength of samples with H2SO4 is generally higher than without.
- Specific impact strength of all samples with H2SO4 is higher than without (2 mm size).
- Common impact strength of all samples with H2SO4 is worse than without.
These results mean that our product after H2SO4 injection is somehow better than before when in static condition, i.e. in storage, but is worse during transportation, when erosion effects takes place, resulting in increased customer complaints of dust. We are now thinking of using formaldehyde to solve the problem.
Mark introduces some other solutions: Have you considered applying seeding technology to improve the impact strength? If your customer accepts urea with sulphur or ammonium sulphate, you could also consider adding some ammonium sulphate to the melt. This could be done for part of the urea production by installing Rotoform technology. Another advantage of installing Rotoform technology is that the load on the prilling tower can be reduced to produce larger prills.
Mr Girish Prakash of Tata Chemicals Ltd, Brabala in India asks for some clarifications: Can you provide approximate values for the extra investment per ton for using the pelletising process? Also, as far as I am aware, farming in this part of Asia is mostly by manual means, farm sizes are small, urea application to the crop is mostly by hand and farmers do not prefer large granules for this type of application.
Mark replies: Producing specialties means higher margins. The Rotoform product is harder and contains extra nutrients required by specific crops. The size of the Rotoform product can be chosen freely in a wide range. The Rotoform technology is also very environmentally friendly.
Mr Ahsan Muhammad Sarfraz of Fatima Fertilizer Ltd in Pakistan joins the discussion: My understanding of the problem is that if the moisture and free ammonia contents of prills are kept in range, strength figures would be higher. The FFCL plant is running at 112% load and fines are around 0.2-0.4% with APS around 2.05-2.1 mm using a tuttle bucket and natural draft prilling tower. Crushing strength is around 840~850 g.
Girish asks a question: Crushing strength of prills without formaldehyde or any other type in excess of 800 g seems to be great. Can you please elaborate how you measure the crushing strength?
Mark provides an answer: In our Gallery you can find one simple and easy way to measure crushing strength: One takes ten prills from a certain size distribution (after sieving) and measures the crushing strength. Then one takes the average value. There is more sophisticated equipment also of course.
Mr Malik Sohail of Safco in Saudi Arabia asks for some clarifications: You mention that sulphur/ammonium sulphate can improve the strength, can you tell me the concentration of sulphate in the final product? Also is there any adverse effect of sulphate in product and is there any upper limit of sulphate in product?
Mark replies: AS concentrations typically vary from 20-50 wt-% depending on crop needs. I think the strength already increases at lower concentrations. S and N fertilizer combined give a better crop yield according to several field tests.
| This series of discussions is compiled from a selection of round table topics discussed on the UreaKnowHow.com website. UreaKnowHow.com promotes the exchange of technical information to improve the performance and safety of urea plants. A wide range of round table discussions take place in the field of process design, operations, mechanical issues, maintenance, inspection, safety, environmental concerns, and product quality for urea, ammonia, nitric acid and other fertilizers. |