TGU catalyst poisoning and deactivation
With the aid of a rigorous kinetic model for TGU hydrogenation reactors, incorporating catalyst deactivation mechanisms, designers and operators can forecast the life expectancy of reactor catalyst beds.
With the aid of a rigorous kinetic model for TGU hydrogenation reactors, incorporating catalyst deactivation mechanisms, designers and operators can forecast the life expectancy of reactor catalyst beds.
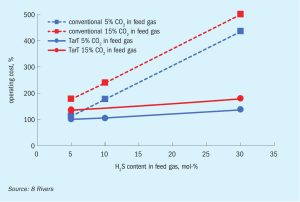
TarT technology, one of 8 Rivers’ decarbonisation technologies, shows promise as an economical, efficient, sour gas sweetening process with near-zero carbon dioxide emissions, and may be key to unlocking access to the world’s sour gas reserves.

Leading companies such as Ballestra, Buss ChemTech, Elessent Clean Technologies (MECS) and Prayon Technologies are working together to solve the numerous production challenges facing the operators of integrated phosphate fertilizer production plants.

Highly efficient ammonia synthesis and subsequent cracking to hydrogen are key processes in the transition to the green hydrogen economy. Catalysts play an important role in the ammonia cracking process. Clariant offers both nickel and precious metal catalysts for this application and research on robust catalysts that allow lower temperatures for increased energy efficiency is ongoing.

In this review article, Hatch’s Jayden Ladebruk, Lyndsay Tran, Amelia Parrenin, and Edward DeRose outline the wide range of phosphoric acid production technologies, and discuss how industry challenges are influencing the choice of phosphoric acid process.
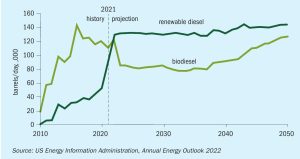
The production of renewable fuels by retrofitting existing refineries and their infrastructure is witnessing exponential growth. The impact on the existing amine, sour water and sulphur recovery units is inevitable. Based on several case studies, Worley Comprimo discusses the various options available to holistically review the sulphur block to determine the impact and mitigation of processing bio-feed.
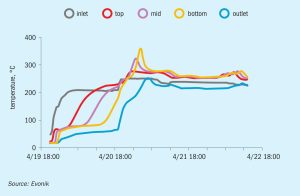
Evonik explores the development of catalyst reuse and how recycled hydroprocessing catalysts can be successfully applied in tail gas treating units to deliver cost and performance gains.

Incorporating sulphur into polymers is known to improve their properties. New research is looking into the varied world of sulphur co-polymers, unlocking new materials for batteries, structural applications and clean technology.

Sulphur Experts Inc. combines new learnings, historical data, and recent onsite experience from operating companies to show what factors really impact ammonia plugging risk and what can be done to control them in order to allow for a wider operating range for SWS processing in the refinery SRU.

Advances in technology, equipment and reagents are enhancing phosphate fertilizer production. Optimisation of standard equipment is also vital for ensuring process efficiency.