Nitrogen+Syngas 397 Sep-Oct 2025
12 September 2025
Increased efficiency through advanced gauze geometries
AMMONIA OXIDATION CATALYST
Increased efficiency through advanced gauze geometries
The development of ammonia oxidation catalysts is challenging due to the need for precise control over catalyst properties to achieve high efficiency and stable process operations. Additionally, the process requires extensive research and advanced characterisation techniques to understand and optimise the reaction mechanisms, making the development procedure complex and resource-intensive. Dr. Florian Knaus and Dr. Christian Goerens present Umicore’s development and potential of a novel catalyst for the Ostwald process, aiming to enhance efficiency and sustainability in high-pressure nitric acid production.
The Ostwald process forms the basis for the production of around 80 million t/a of nitric acid (HNO3), and is the primary industrial method for synthesising this compound1.
Nitric acid is a crucial nitrogen source, extensively used in the manufacture of fertilizers, explosives, and various chemicals. The Ostwald process involves three key stages: the catalytic oxidation of ammonia (NH3) to nitric oxide (NO), the subsequent oxidation of NO to nitrogen dioxide (NO2), and the absorption of NO2 in water to form nitric acid. Catalysts, typically gauzes made from platinum group metals (PGMs) by various configurations, facilitate the oxidation of ammonia. This process also generates byproducts such as nitrogen (N2) and nitrous oxide (N2O), a significant greenhouse gas. The nitric acid industry, with an annual by-production of around 400,000 t N2O, is the second-largest emitter of nitrous oxide, following the agricultural sector2.
The development of novel catalysts for the Ostwald process is driven by the need to meet evolving industrial demands as well as increasing environmental standards. While significant progress has already been made over the past century, further advancements now require smaller, incremental improvements that demand substantial effort. By integrating advanced simulations, precise prototyping, and rigorous experimental validation, Umicore ensures that these high-effort steps lead to innovative and effective catalytic solutions.
Kinetics of the Ostwald process
To investigate optimised catalysts for the Ostwald process, it is essential to first review the complexity of the process. In several studies a combination of flow characteristics and surface reaction mechanisms is used for simulation3,4. This approach is necessary since the design of the catalyst and the resulting mass transfer within the gauze package influences the concentrations on the catalyst surface and the kinetics of the ammonia oxidation.
A kinetic model used in current investigations is based on the mechanistic model developed by Kraehnert and Baerns. This was selected due to its superior agreement between experimentally observed and simulated product formation rates5. Fig. 1 summarises the mechanistic scheme and kinetic parameters employed for the ammonia oxidation reaction.
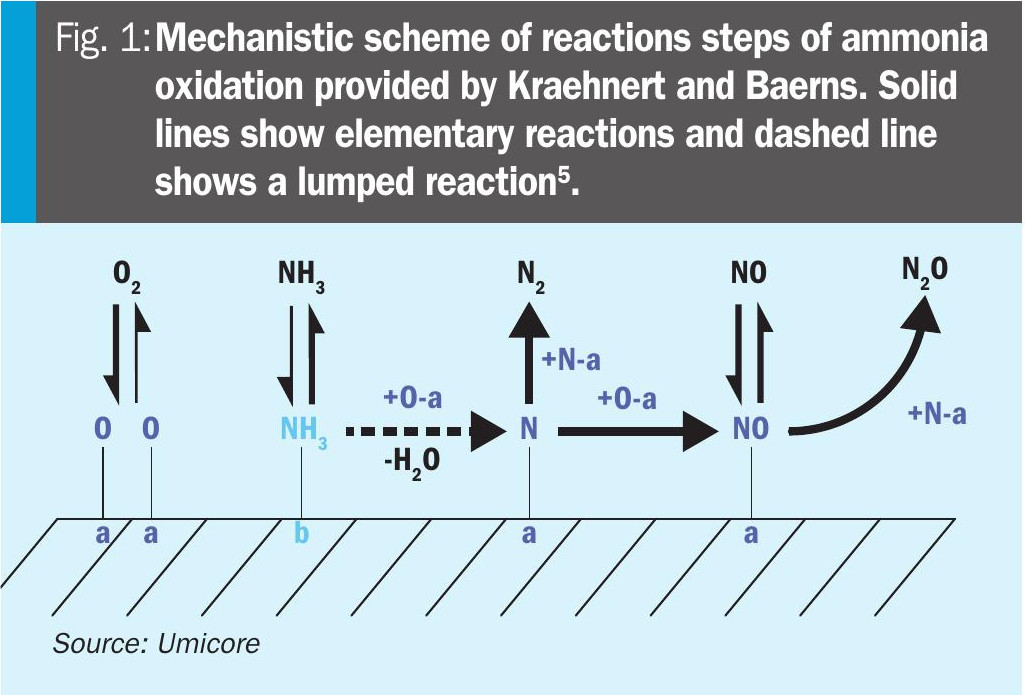
The model comprises ten elementary reactions involving six gas-phase species and six surface-bound intermediates. Adsorption steps for oxygen, ammonia, and nitrogen monoxide are treated as reversible, whereas adsorption of the by-products nitrogen and nitrous oxide is excluded.
Differentiation of plant types in the Ostwald process
The industrial production of nitric acid based on the Ostwald process, which involves the catalytic oxidation of ammonia, subsequent oxidation of nitrogen monoxide, and absorption of nitrogen oxides in water to form nitric acid. The implementation of these steps varies depending on the operating pressure, which defines the type of plant configuration. Three main categories of plant types are distinguished: low-pressure, medium-pressure and high-pressure plants6.
Low-pressure plants operate at pressures between 1 and 2.2 bar and represent the earliest generation of nitric acid production facilities. These plants typically perform both ammonia combustion and NOx absorption at atmospheric or near-atmospheric pressure. While simple in design, they suffer from low absorption efficiency and high NOx emissions, making them largely obsolete under current environmental regulations.
Medium-pressure plants, operating between 2.3 and 6 bar, offer a balance between process efficiency and investment cost. They are widely used for small to medium production capacities and are particularly economical in regions with moderate energy and feedstock costs. Modern facilities employ dual-pressure configurations, which combine the medium-pressure ammonia oxidation with a high-pressure absorption (9 to 14 bar). This design optimises both (reaction kinetics and absorption efficiency), offering improved energy balance, lower NOx emissions without extensive tail-gas treatment, and high product yields with reduced ammonia consumption. Dual-pressure plants are particularly favoured in Europe due to their superior performance in terms of energy efficiency and environmental compliance.
High-pressure plants, operating between 7 and 13 bar, are favoured in regions such as North America where energy and feedstock costs are relatively low. These plants compress both air and nitrous gases to high pressures, enabling enhanced absorption efficiency and reduced equipment size. The high-pressure operation allows for significant steam generation, which can be used for power recovery or exported. However, the increased pressure also leads to higher catalyst losses and requires robust materials and design to withstand the demanding operating conditions. High-pressure plants often incorporate interstage cooling in compressors and advanced heat recovery systems to optimise performance.
In summary, the choice of plant type is influenced by regional energy costs, environmental regulations, and desired production capacity, with dual-pressure plants representing the current state-of-the-art in nitric acid production technology.
Development of novel catalyst designs
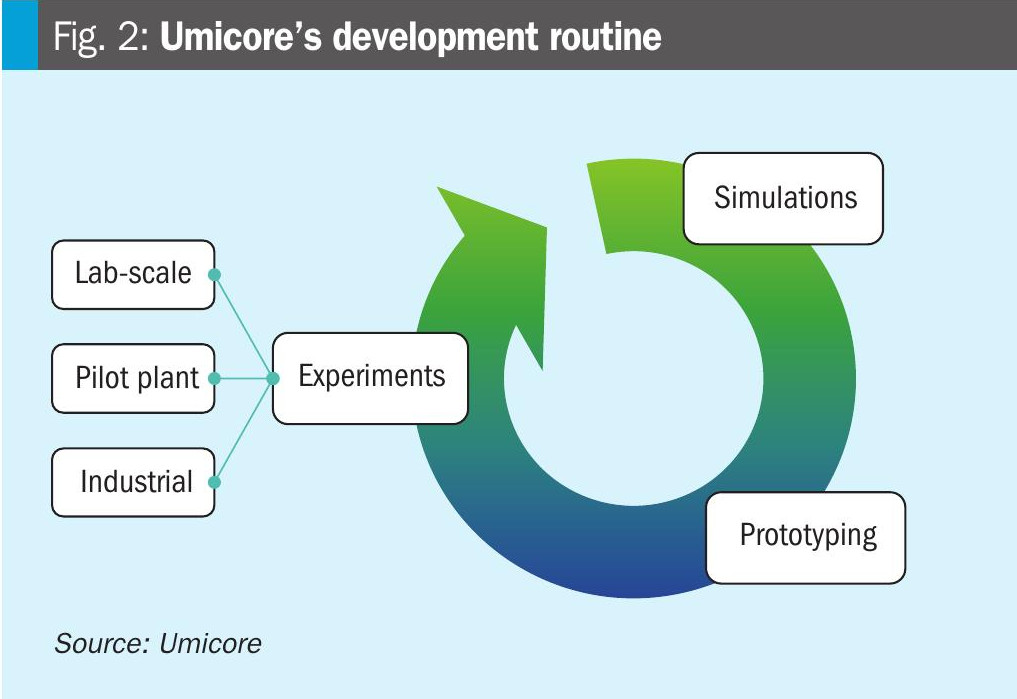
At Umicore, the development of new catalysts involves a synergistic approach combining advanced simulations, prototyping, and experimental validation (Fig. 2). This methodology enables the design and optimisation of high-performance catalysts. Computational models predict catalyst behaviour, while prototyping and rigorous testing ensure theoretical predictions align with real-world applications. This integrated process accelerates development and ensures innovative catalytic solutions.
Simulation
Simulations are essential in the development of catalyst gauzes. They enable the prediction of material and design performance without the need for extensive and expensive experiments. This approach helps identify and optimise the most promising options, enhancing efficiency and durability. As a result, the development process is accelerated, leading to the creation of more effective and innovative catalyst gauzes.
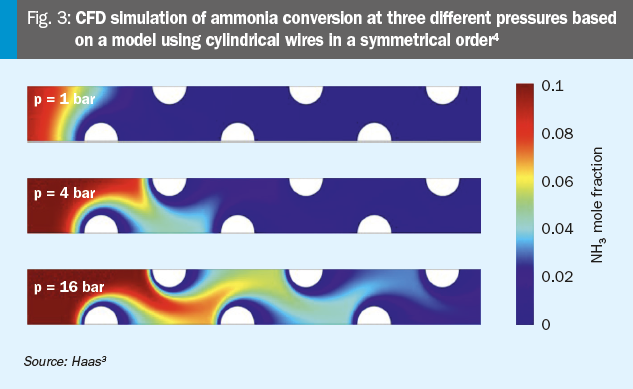
The following investigations are made with the CFD model by Haas3. The model is based on a multi-wire catalyst geometry. The setup includes ten staggered rows of cylindrical wires. The main flow is in the x-direction. Due to symmetry, only half of the wires are modelled in an axial section. The simulations assess how radiation and pressure influence the catalyst performance. One outcome of simulations is that at higher pressures, the reaction tends to shift towards the lower gauzes. This behaviour is observed due to the increased density and reactivity of the gases, which enhances the interaction with the catalyst surfaces located at lower positions:
This shift in reaction towards the lower gauzes at higher pressures could be attributed to specific reaction parameters. These factors may lead to lower efficiency in the catalytic process, as the upper layers of the gauzes are not being fully utilised. Consequently, it might be important to increase the reaction surface on the top layers to enhance overall efficiency and ensure a more uniform distribution of the catalytic activity.
Prototyping
Prototyping plays a crucial role in the development of new catalysts at Umicore, and the use of flatbed knitting machines is an integral part of this process. These machines allow for the precise and efficient creation of catalyst gauzes, enabling a quick manufacturing and testing of various designs. By leveraging the capabilities of flatbed knitting machines, Umicore can optimise the structure and composition of catalyst gauzes, ensuring they meet the desired performance criteria. This approach not only accelerates the development cycle but also enhances the overall quality and effectiveness of the catalysts.
With simulations and Umicore’s overall established development routine, a novel gauze type was developed which shows a higher wire density compared to a standard gauze geometry. It is expected that this increased surface leads to a higher conversion in the upper gauze layer, especially in high pressure plants.
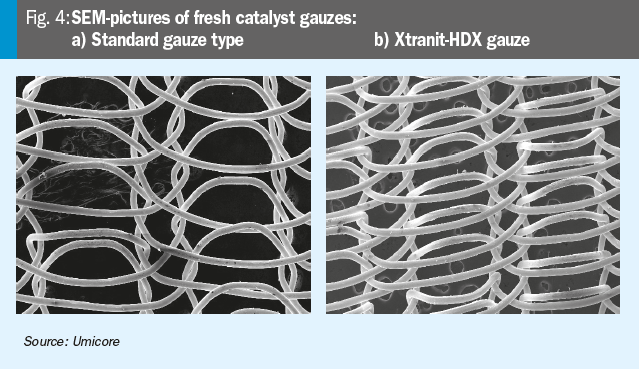
In Fig. 4 a novel gauze type (4b) is shown in comparison to a standard gauze (4a), which has an increased surface to increase the conversion in the upper gauze layer, especially in high pressure plants.

As indicated in the graph, the novel Xtranit-HDX gauze type increases the catalyst performance towards higher conversion efficiencies of ammonia to NOx significantly.
Experiments
Besides simulations and developing prototypes, the final evaluation of novel catalysts can only be done in real experiments. For the following tests, the pilot plant at INS Puławy is used. The institute is equipped with state-of-the-art facilities and advanced analytical tools that enable comprehensive evaluation of catalyst prototypes. Additionally, it can run three reactors in parallel to get a direct comparison between different types of catalysts.
The results of pilot plant tests at high pressure conditions with varying plant load are shown in Fig. 5. The parameters used in the pilot plant to test the novel Xtranit-HDX gauzes are shown in Table 1.
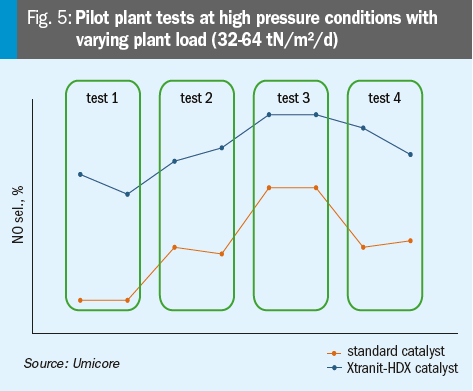
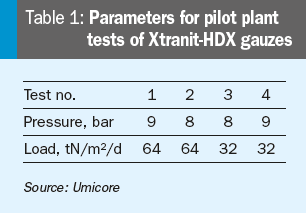
Besides pilot plant tests, first industrial tests were also carried out: For testing purposes, this novel type of catalyst design was installed in a high pressure plant with a load of 70 tN/m²/d and a pressure above 10 bar.
In Fig. 6 a comparison of two standard catalyst packages and two catalyst packages containing Umicore’s Xtranit-HDX catalyst gauzes is shown.
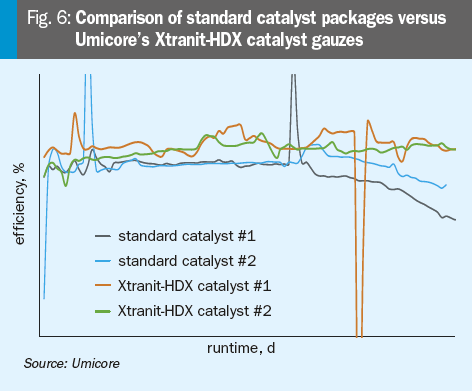
By reviewing this comparison, two main observations are shown: First, the catalyst packages containing the novel Xtranit-HDX gauzes indicate an overall higher efficiency over the whole campaign. Besides that, an increase in long term stability can also be observed.
These first results from an industrial application show impressively how the adjustment of the knitting structure in the novel Xtranit-HDX gauzes can positively influence the performance of Umicore’s catalyst especially in high pressure plants.
Summary
Umicore’s development of a novel catalyst for the Ostwald process is driven by the need to meet evolving industrial demands and environmental standards. Significant progress has been made over the past century, but further advancements now require smaller, incremental improvements that demand substantial effort. To achieve these improvements, Umicore integrates advanced computational simulations, precise prototyping, and rigorous experimental validation.
Simulations provide a predictive framework for understanding how potential catalysts will behave under various conditions, streamlining the selection process by identifying the most promising candidates. Prototyping involves creating physical models of the selected catalysts, bridging theoretical predictions with real-world applications. Rigorous experimental validation assesses the performance, stability, and scalability of the prototypes, ensuring that the catalysts produced meet the highest standards of quality and efficiency.
The goal is to enhance efficiency and sustainability in high-pressure nitric acid production. By focusing on high-pressure plants, this catalyst aims to optimise the reaction conditions, reduce energy consumption, and minimise environmental impact, ultimately contributing to more sustainable industrial practices.
Outlook
Looking ahead, Umicore continues to focus on advancing catalyst technology to meet the evolving demands of the industry and environmental standards. By participating in the governmental funded project SuNiPro (Sustainable Nitrates Production) Umicore aims to further enhance the sustainability and efficiency of nitric acid production. These initiatives reflect Umicore’s commitment to continuous improvement and innovation in catalyst development for the Ostwald process.
Umicore will be sharing further insights at the upcoming Nitrogen + Syngas conference in Barcelona (10-12 February 2026) and the ANNA conference in Omaha (12-17 October 2025).
References