Nitrogen+Syngas 397 Sep-Oct 2025

11 September 2025
Incident No. 7 Nitric acid tank explosion – Part 1

The following case study describes a serious incident and the consequences of erroneously mixing nitric acid with hydrochloric acid. In Part 1 we report on the incident and the causes that led up to the event. In part 2 the impact, recommendations and lessons learned will be discussed.
The first news report
It was reported that an explosion and a large orange toxic cloud erupted on Friday 4 July 2025 at a factory (not related to fertilizers) after a chemical reaction between two hazardous substances inside a truck. No one was injured, but authorities evacuated the plant and closed roads as a precaution.
The incident began when nitric acid and hydrochloric acid – corrosive chemicals used to clean equipment – came into contact inside the vehicle. A spokesperson said the cloud contained dangerous substances that could irritate skin and airways. “Measurements by the fire service showed that the smoke contained toxic substances,” the spokesperson said. However, the chemicals quickly dispersed into the air without settling on the ground. Around 1 am, the cloud had evaporated and dissolved, according to the safety region. The mayor later announced that the situation was “fully under control.”
Authorities issued an alert advising residents nearby to close windows and doors and turn off ventilation systems. Several houses are located near the site. Emergency services closed access roads, urged people to stay away from the factory grounds, and evacuated the plant as a precaution.
The police cordoned off the area as a crime scene and launched an investigation to determine how the acids were able to mix. “The cause of the incident will be carefully investigated,” the mayor said. Officers are examining all possible scenarios, including deliberate tampering or an operational error.
Background information
The reaction between nitric acid (HNO3) and hydrochloric acid (HCl) forms aqua regia, a highly corrosive mixture capable of dissolving noble metals like gold and platinum. This mixture is primarily composed of nitric acid, hydrochloric acid, nitrosyl chloride (NOCl), chlorine gas (Cl2), and water.
Aqua regia is formed when concentrated nitric acid and hydrochloric acid are mixed (typically in a 1:3 ratio). The reaction is not a simple acid-base neutralisation. Instead, it involves oxidation and complex formation. Nitric acid acts as an oxidising agent, converting some chloride ions (from HCl) into chlorine gas. The chlorine gas then reacts with nitric acid to form nitrosyl chloride. The reaction can be represented as:
HNO3 + 3HCl → NOCl + Cl2 + 2H2O
Aqua regia is historically significant for its ability to dissolve gold, leading to its name meaning “royal water”. It’s used in various applications, including refining precious metals, cleaning glassware, and as a reagent in chemical synthesis. Due to its hazardous nature, it is crucial to follow strict safety protocols when handling aqua regia.
Properties of aqua regia are listed below:
- Corrosive: Aqua regia is highly corrosive due to its strong oxidising and acidic properties.
- Fuming: It produces irritating and toxic fumes, primarily chlorine and nitrosyl chloride.
- Oxidising: Aqua regia is capable of oxidising metals, including gold and platinum, which are normally resistant to individual acids.
- Dissolves metals: The reaction of aqua regia with gold (Au) and platinum (Pt) involves the formation of soluble chloroauric acid (HAuCl4) and chloroplatinic acid (H2PtCl6) respectively.
- Decomposition: Nitrosyl chloride (NOCl) can further decompose into nitric oxide (NO) and chlorine gas.
- Dangerous: Aqua regia is extremely hazardous and should be handled with extreme caution in a fume hood, using appropriate personal protective equipment (PPE).
The following information was provided in the company’s preliminary investigation report dated 28 August 2025.
The incident
The incident began on 4 July 2025 at 12:58 pm when a tanker carrying hydrochloric acid (30% solution) from a third party supplier arrived at the wrong location. Drivers were allowed onto the scene due to poor communication and lack of verification. Without checking the bill of lading or the UN code, the hydrochloric acid was discharged into the tank containing the nitric acid solution (25% solution) at 1:20 pm. The error was discovered by the logistics department around 3:30 pm. The crisis group was activated and after consultation with a dedicated external company, it was decided to pour the contents of the tank into a specialty chemical container (IBC). Due to the rapidly changing situation, this ultimately didn’t continue. Around 7:14 pm the first sign of a chemical reaction appeared: orange smoke rising near the gas scrubber. Around 9:10 pm the scene was evacuated and the fire brigade was put into service. The situation escalated rapidly and at 10:13 pm the tank exploded.
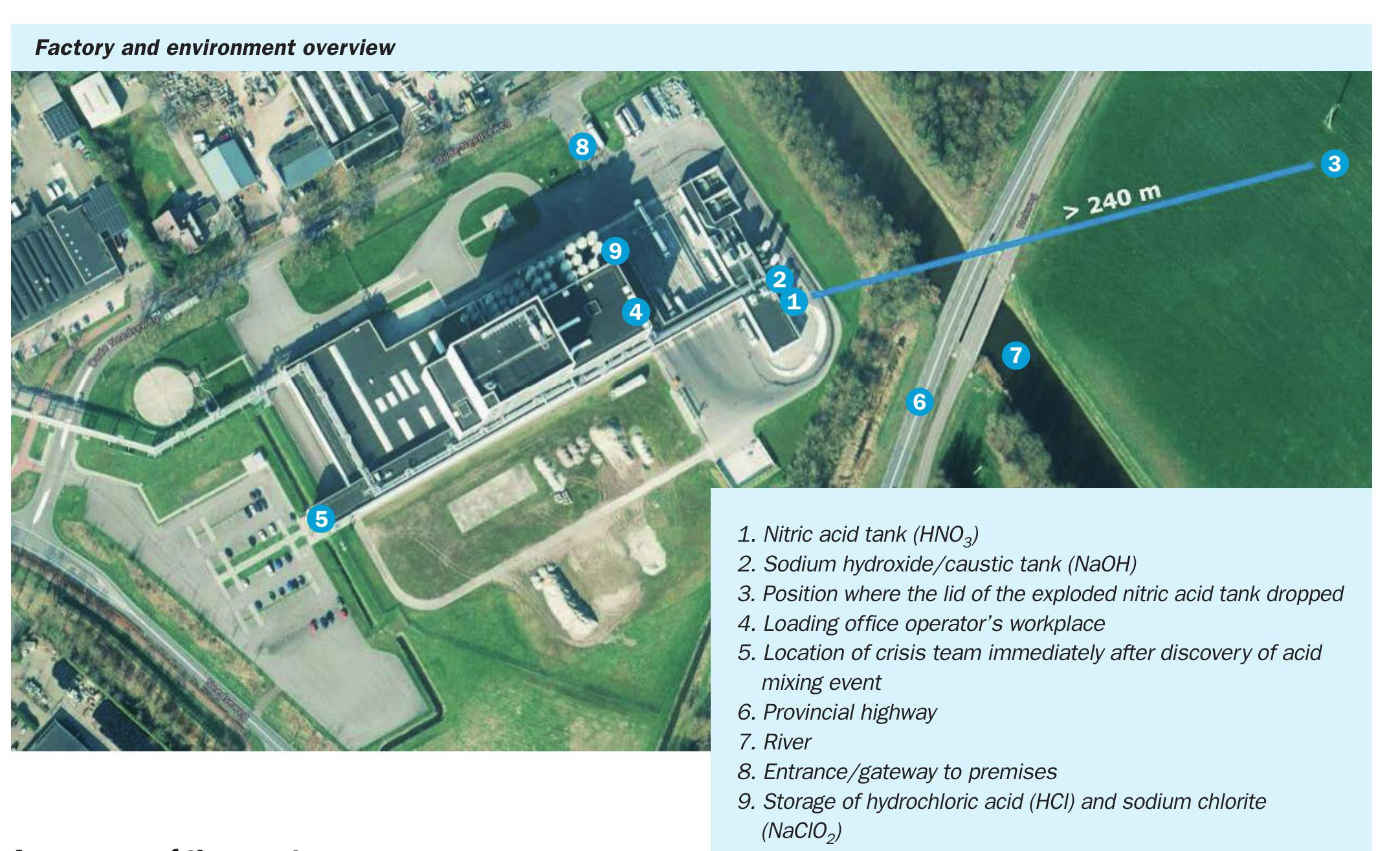
A summary of the event:
- Chemical reaction – Hydrochloric acid was accidentally added to the nitric acid tank, resulting in a violent chemical reaction.
- Gas formation and explosion – reaction resulting in corrosion, pressure increase and orange smoke column; Later, the tank exploded and the lid was thrown hundreds of metres.
- Smoke spread – Smoke gradually spread with the wind causing visible disturbance to the surrounding area.
- No casualties but damage – No casualties but smoke and noise disturbance causing damage to the environment.
- Stopped production – factory closed.
The causes
Due to a series of human errors, hydrochloric acid (30% solution) was accidentally discharged into a storage tank containing nitric acid (25% solution). Both of these substances are used in the plant for cleaning and water purification. The mixing of the two acids resulted in a drastic chemical reaction, and preliminary findings suggest that both human and technical and organisational factors played a role. The driver delivered his goods to the wrong production site and was allowed into the factory. The identity of chemicals was not verified by bills of lading or signs such as UN codes. The UN code refers to an orange sign with a black number that indicates which hazardous substances are being transported. The UN code identifies substances on tanker trucks and tank units. The scheduling was misinterpreted and the prescribed verification procedure was not applied. In addition, the driver used the connector he carries with him to adapt the hose of the hydrochloric acid tanker to the nitric acid tank, and connected it in front of the staff present. The highly reactive combination of hydrochloric acid and nitric acid led to rapid corrosion of the tank and ultimately explosion from heating the liquid in the sealed tank. So far, it has been found that with the training available, operators cannot easily cope with this situation, instructions are scattered across multiple systems, and the four-eyes principle is not applied when connecting and unloading chemicals.

While the crisis team acted quickly based on the local company’s contingency plan, the case of chemical mixing was not covered. The causes are summarised as follows:
- Wrong location and insufficient verification – The driver delivered to the wrong factory and did not check the identity of the chemical via bill of lading and UN code.
- Incorrect connection – A hose was connected to the wrong tank using a carry-on connector in the presence of employees.
- Organisational improvement points – Operators have been found to be unable to handle the situation easily to date. Indications are fragmented and the four-eyes principle is not applied.