Nitrogen+Syngas

7 August 2025
Problem No. 52: High nickel content in high pressure urea synthesis
The detection of active corrosion in a urea plant is difficult. Local corrosion can hardly be detected unless leak detection systems or conductivity measurements in steam/condensate systems are used. Overall corrosion however can be detected in the warehouse by checking for discoloration (reddish colour) of the product or via analyses of the urea final product.
In many plants, product analyses are used to verify the condition of the plant. An element that is often analysed is nickel because it is a reliable parameter to verify local active corrosion in a urea plant. Nickel is present in the stainless steel that protects the pressure vessels and as an alloying component in the HP piping. Since there is ongoing corrosion in the synthesis section, there is always some nickel present in the final product.
How much nickel can be tolerated cannot be easily stated and varies from plant to plant. The type of alloy protection in HP synthesis sets the fingerprint of the amount of nickel that will be found. In order to have an indication about what is going on in your urea plant it is important to look at trends; in case of active corrosion one will observe a linear increase of the nickel content. Of course in case of increasing nickel in the product adequate actions are needed. That means shut down the plant, eliminate the source and re-passivate.
Krzysztof Czachór from Grupa Azoty Zakłady Chemiczne Police in Poland starts the round table discussion: I would like to ask what the standard nickel content measured in the high pressure (HP) urea synthesis section is? When should we be worried about corrosion? In which vessels do you check the nickel in your plants? We are worried because the nickel content in our HP scrubber outlet has increased compared to previous years and is higher than the standard level. What could the reason be for this?
Mark Brouwer of UreaKnowHow.com replies: Nickel is measured to check for active corrosion. In case of active corrosion it cannot become passive again unless the plant is shut down, drained and then re-passivated. Look for increasing trends, not peaks, then you should know what nickel levels are normal and if there is an increasing trend. It does not make a lot of sense to make comparisons with other plants as it depends on, for example, the materials applied. For example, 316L UG and 25-22-2 have completely different nickel contents.
Krzysztof replies: Thank you for quick reply. We decided to increase the amount of oxygen in the urea synthesis from 0.8 to 1.1 vol-% and noticed that passive corrosion started to decrease. Do you have any advice what could have happened?
Mark asks for further information: For which equipment did the passive corrosion decrease? How did you conclude that passive corrosion rates decreased when you increased the oxygen content? Are we talking about a Stamicarbon CO2 stripping plant with falling film high pressure carbamate condenser (HPCC)?
Krzysztof replies: Yes, it’s a Stamicarbon CO2 stripping plant with falling film HPCC. We noticed that the nickel level started to decrease in the HP scrubber, but remained stable in other equipment. We started to measure the nickel level in the HP carbamate pump and noticed that it was high. Can this be the reason for a high Ni level in the HP scrubber? Do you measure the Ni level in the LP section as well?
Mark responds: The HP scrubber has one has relatively high partial oxygen levels in the gas phase, so one would not expect that going from 0.8 vol-% (which is already high) to a higher figure would give benefits in the HP scrubber. One should look at trends as the analysis is not very accurate. For example, what change in nickel level did you observe over the HP scrubber at 0.8 and 1.1 vol-% oxygen? Did
you look at this trend? Yes, one can also measure nickel in the LP section as the stainless steel there is also passivated by the oxygen.
Krzysztof provides some data: Please see the nickel content trends for the synthesis and HP carbamate pump in the figures. We increased the oxygen content on 19th October. Any conclusions?
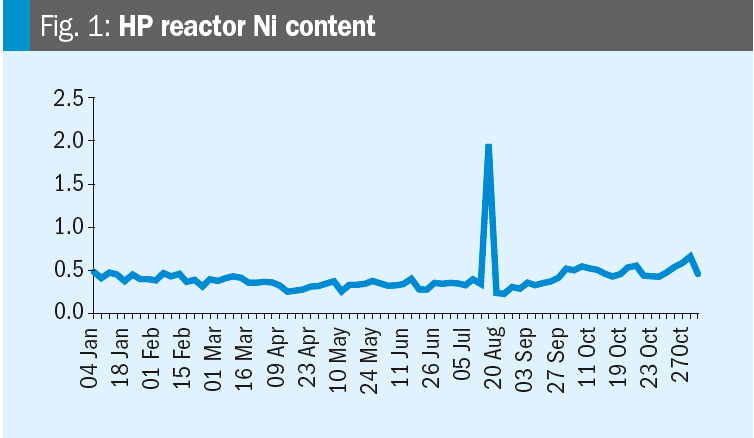
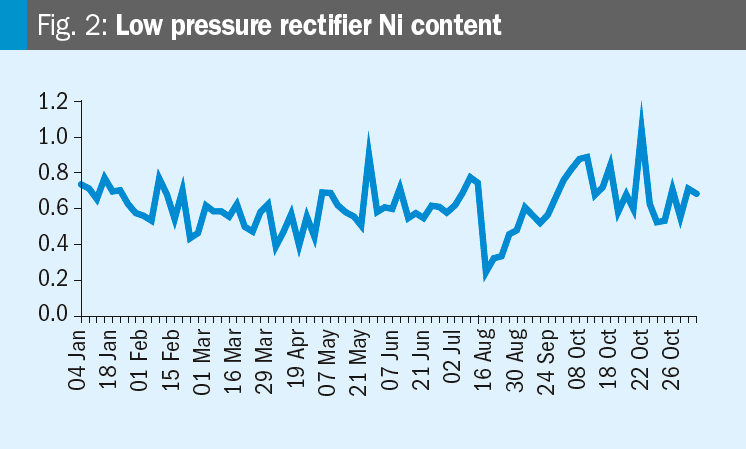
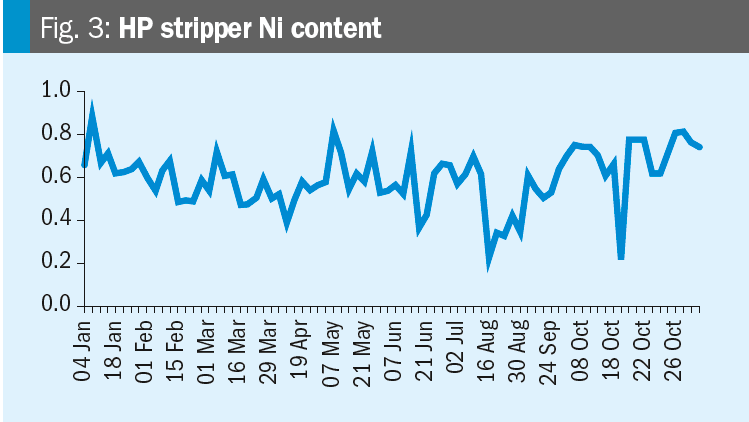
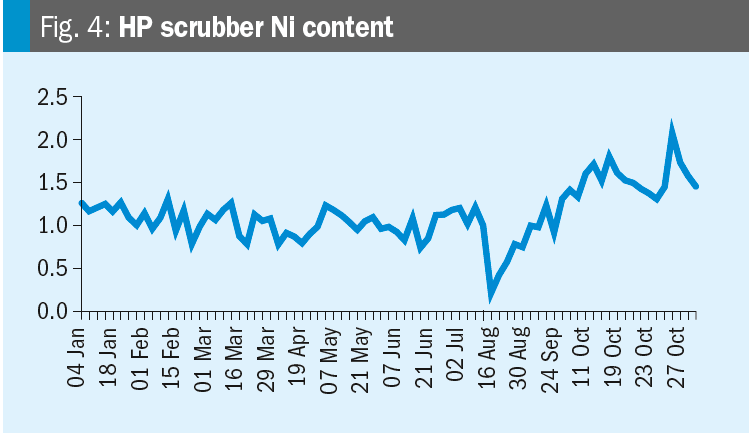
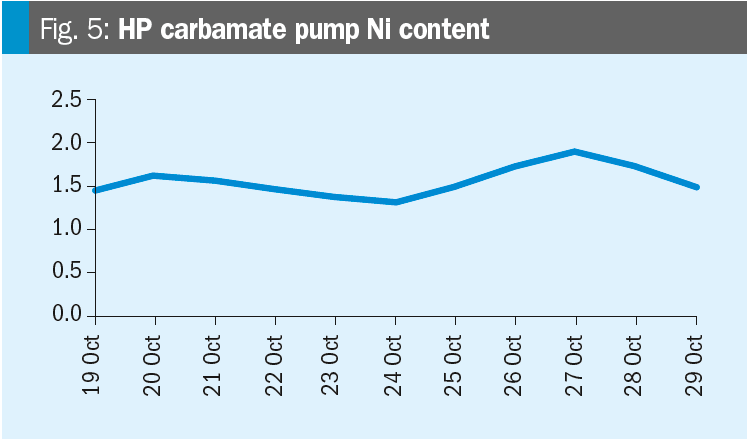
Mark replies: Further discussions lead to the conclusion that the changes in nickel are likely related to turnaround activities (the T/A was from July 12 to August 16). After a turnaround it is very likely that nickel contents increase due to grinding and other work in the synthesis section. When cleaning is not perfect this material will dissolve in the ammonium carbamate and higher nickel levels will occur.
Krzysztof concludes: Thank you for your constructive discussion and for sharing the experience of you and your colleagues. It has helped us to understand what could have happened.
Related to this topic is another round table discussion below which asked the question: Do You know why only nickel provides trustworthy information for active corrosion?
Gholam Moazzez of Khorasan Petrochemical Company in Iran replies: The passive layer consists of chromium complexes; therefore if the passive layer fails for any reason, the chrome of the grain boundaries remains and contributes to the chromium complexes on the surface of the liner and nickel is soluble in product.
Ahmed Hegazy of MOPCO in Egypt gives his opinion: As urea-formaldehyde forms a violet-colored nickel salt of diphenylcarbazide and iron or chromium do not, this provides good evidence for nickel analysis from alloy in vessels of high pressure synthesis.
Norozipour of Khorasan Petrochemical Company in Iran adds: I think chromium and iron are insoluble in urea solution, but nickel is soluble in this solution which makes it a suitable indicator for active corrosion in urea plants. In this case iron and chromium precipitate in
the exchangers and vessels and cause fouling of the equipment.
| This series of discussions is compiled from a selection of round table topics discussed on the UreaKnowHow.com website. UreaKnowHow.com promotes the exchange of technical information to improve the performance and safety of urea plants. A wide range of round table discussions take place in the field of process design, operations, mechanical issues, maintenance, inspection, safety, environmental concerns, and product quality for urea, ammonia, nitric acid and other fertilizers. |


