Nitrogen+Syngas 334 Mar-Apr 2015

30 April 2015
Problem No. 29: Stripper versus decomposer: What is the difference?
In 1967, Mr Piet Kaasenbrood of Stamicarbon invented the CO2 stripper. The CO2 stripper revolutionised the urea process industry as it reduced the synthesis pressure from about 200 bar to 140 bar and reduced the energy consumption of a urea plant by about 50%. The CO2 stripper decomposes the unconverted ammonium carbamate coming from the reactor at the same pressure as that in the reactor. Therefore no water needs to be added to recycle this carbamate to the reactor, which is beneficial for the conversion in the reactor.
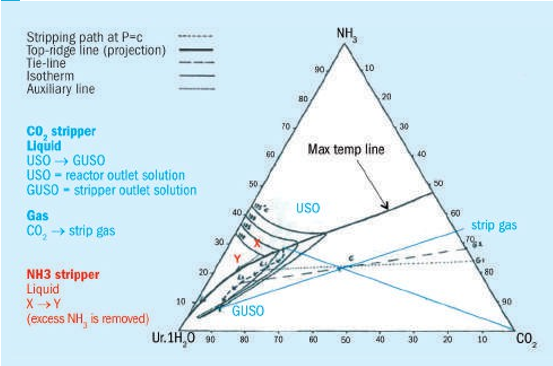
The phase diagram of the system NH3-CO2-Urea-H2O (see picture) shows that by stripping with CO2 one is able to significantly reduce the ammonia content in the reactor outlet while at the same time reducing the temperature. Minimum temperatures are vital to keep corrosion rates under control and to allow economical materials of construction to be used. A decomposer on the other hand only removes the excess ammonia from the reactor solution until the azeotropic composition is reached. During this process the temperature increases. It is for this reason that in a CO2 stripper with a bottom temperature of 170-175°C 25-22-2 stainless steel can be used, while in a Saipem stripper, with a bottom temperature of 204-212°C, titanium or zirconium needs to be applied to handle the high temperature.
Mr Kessla Belkacem of AOA in Algeria initiates the Round Table discussion: I have a question: I would like to know what the difference is between decomposing and stripping?
Mr Mark Brouwer of UreaKnowHow.com in the Netherlands replies: These terms are frequently mixed up. Theoretically, stripping is decomposing with the help of a stripping gas. So a CO2 stripper works with CO2 as a stripping agent and is a stripper. An NH3 stripper is in my view more a decomposer than a stripper although one could argue that the decomposed carbamate acts as a kind of stripping agent.
Mr Malik Sohail of SAFCO in Saudi Arabia gives his view: Actually the purpose of both the stripper and decomposer is the same, the decomposition of ammonium carbamate. If this decomposition takes place mainly by partial pressure motive then it is called stripping and if this motive is heat then it is called decomposing.
Mr Nawal Gupta of KRIBHCO in India contributes to the discussion: If ammonium carbamate is dissociated into NH3 and CO2 gases by reducing the pressure of the solution, it is called decomposition. If the same is achieved by reducing the partial pressure of one of the components (that of NH3 in the case of CO2 stripping or that of CO2 in the case of NH3 stripping), it is called stripping.
Mr Kashif Naseem of SABIC in Saudi Arabia provides his view: Decomposition: Carbamate is formed in the reactor and passes to the stripper. With heat of steam this reaction is reverted back to ammonia and CO2 with breakage of its bond.
Stripping: The formed ammonia and free ammonia are forced to come out using CO2 as the stripping agent. Mr Thota Raju of Nagarjuna Fertilizers and Chemicals Ltd. in India adds to the discussion: In my view we normally heat the carbamate solution to reverse the reaction towards the reactant side (i.e. NH3 and CO2). The NH3 and CO2 will be in the liquid phase and in order for them to go to the gas phase their saturation pressure at those conditions should be greater than partial pressure of the respective in gas phase. In a stripper the gas phase pressure is high so suppose that in a Saipem process the excess ammonia and formed ammonia is present in a higher quantity (i.e. high mole fraction) than as per the vapour liquid equilibrium, it will start vaporising. It first increases its partial pressure in the gas phase and since the system pressure is constant it decreases the CO2 partial pressure then stripping starts.
Mr Azad Panchal of GNFC in India asks a similar question: In the Saipem urea plant the stripper has the same function as the MP decomposer, why is one called a stripper and the other a decomposer?
Mr Nimesh Maurya of KRIBHCO in India replies: In the HP stripper the decomposition is carried out by stripping action using Henry’s law in the presence of excess ammonia while in the medium pressure decomposer decomposition is carried out by elevated temperature at reduced pressure only. There is not any stripping action.
Azad comments: But the stripper is also operating at high temperature by using medium pressure steam and at a reduced pressure of 145 kg/cm2, while the reactor operates at 160 kg/cm2.
Nimesh replies: In the HP stripper part of the ammonium carbamate is decomposed due to elevated temperature, but the decomposition is mainly achieved by stripping action so it is called a stripper.
Mr Shashank Sharma of Tata Chemicals Ltd. contributes: In the stripper, 80% of the ammonium carbamate is decomposed into NH3 and CO2 by the stripping action of NH3 vapours occurring due to Henry’s law as the NH3 is present in excess. Since the main action here is decomposition by stripping it is called stripper. In the MP decomposer the decomposition takes place due to flashing and the increase in temperature of the ammonium carbamate solution in the medium pressure section, as a result of which it is decomposed into NH3 and CO2.
Mr Prem Baboo of NFL in India gives a detailed reply: Definition of a stripper: To reduce the partial pressure of the product by swamping the system with one of the reactants, which considerably reduces the partial pressure of the other reactant without changing the total pressure. Either CO2, NH3 or both can be used as a stripping agent.
Definition of a decomposer: The process based on the first principal of decrease in pressure and increase in temperature followed by a series of decomposition stages where the reactor discharge is treated successively at lower pressure.
The delta P for a decomposer is greater than for a stripper. There is no delta P between the reactor and the stripper, however in a Saipem process there is small delta P.
With the decomposer the solution flashes due to the large difference of pressure and with the stripper the base is Henry’s law of partial pressure and mass transfer is also involved.
Decomposition is based only on high temperature and low pressure, decomposition occurs due to let down, the water recycle is greater. Stripping is based on the partial pressure. In the Stamicarbon process CO2 is introduced in the stripper, which is advantageous because CO2 increases P1 CO2 to P2 CO2, so P1 NH3 will be reduced to P2 NH3 to maintain constant total pressure as PCO2+PNH3=Total Pressure. Now K-1 = (X2NH3 X XCO2) / XCarb. at particular temp K1 is constant so when XNH3 is reduced to keep K1 constant X Carbamate will be reduced much faster by decomposition as XNH3 appears in the equation with power of 2.
Decomposition is favoured by low pressure but energy is required to recycle the decomposition products back to the reactor. Also, at low pressure more water is evaporated during decomposition and this water will enter the urea reactor along with the recycle stream and adversely affect the conversion. Thus if the decomposition is carried out in a single stage near atmospheric pressure the carbamate formed during recovery at the same pressure will carry a lot of water. Considering these factors, decomposition is carried out in a number of stages.
In a stripping process the first stage decomposition and recovery is done at the reactor pressure which permits heat to be recovered at high pressure and also results in energy saving the for returning the recycle streams to the reactor. However, in the Saipem process there is a difference in pressure between the reactor and stripper, so there is the additional advantage of differential decomposition.
Mark adds to Prem’s explanation: The phase diagram above helps to explain the difference between the CO2 stripper and NH3 stripper (or HP decomposer).






