Fertilizer International 529 Nov-Dec 2025

21 November 2025
Innovative cadmium and arsenic removal from phosphoric acid
DECADMIATION TECHNOLOGIES
Innovative cadmium and arsenic removal from phosphoric acid
In this article, John Carr, Lei Zhang and Yu Zheng of Syensqo review the various decadmiation techniques currently available. They introduce Syensqo’s ACCO-PHOS® reagent range – and present recent improvements to this technology that increase removal efficiency for heavy metals such as cadmium and arsenic from phosphoric acid.

Rapeseed (Brassica napus) field, Germany. In the EU, fertilizers applied to fields like this must contain less than 60 mg Cd /kg P2O5.
Introduction
The demand for phosphate fertilizers and feed phosphates is on the rise, due to a growing global population and higher per capita calorific intake. The phosphate rock used in the manufacture of these products can contain cadmium and other heavy metals, often at concentrations that are potentially toxic and mutagenic to humans. Consequently, the introduction of regulatory limits on the heavy metal content of finished fertilizers and feed phosphates – both existing and pending – will increasingly necessitate their removal.
While the toxicity threshold of heavy metals is still the subject of much debate, the EU is currently working towards a phased reduction in permissible cadmium levels in fertilizer products. The 2019 Fertilising Products Regulation (FPR) limits the cadmium content of any fertilizer products placed on the EU market to less than 60 mg/kg P2O5 – a requirement that entered into force on 16 July 2022. Alongside the FPR, fertilizer producers are allowed to declare their products as low cadmium in the EU, if levels are below 20 mg/kg P2O5, under a so-called ‘green label’ scheme introduced in 2021.
Over time, to comply with the FPR, demand for commercially viable solutions capable of removing cadmium from phosphoric acid and phosphate fertilizers is expected to increase. Similarly, tighter heavy metal regulations for feed and food phosphates should drive market adoption of technologies for cadmium and arsenic removal from phosphoric acid.
Currently, although the permissible levels of heavy metals in fertilizers are generally higher in other regions versus EU regulations, some campaigners and environmental groups are pushing for tougher legal limits. Therefore, fertilizer and feed phosphate producers in other parts of the world, by investigating heavy metal removal now, can ensure they understand and scope their options if tighter regulation of heavy metal content is introduced in the foreseeable future.
“Cadmium removal from phosphoric acid and phosphate fertilizers is expected to increase.
Heavy metal removal technologies also offer other benefits. For example, they can provide producers with additional disposal options by lowering the heavy metal content of waste streams. Lastly, producers can potentially gain a competitive advantage by offering low (or no) heavy metal fertilizer and feed products to high-value end markets and customers – in response to rising market demand for more ‘natural’ and ‘healthier’ food options.
Need for heavy metals removal
The heavy metal content of phosphate fertilizers products is dependent on several factors. Their original concentration in phosphate rock, the overall type of production process, and individual processing treatments all have an influence.
Heavy metals are partly removed in waste streams such as phosphogypsum and process water. Yet a significant portion still remains in both the phosphoric acid intermediate and finished fertilizer products. In some North African operations, for example, only 10% of cadmium present in the original phosphate rock is recovered with gypsum waste, leaving the remainder in phosphoric acid1.
Heavy metals like cadmium are toxic to the human body and tend to accumulate in vital organs – where they remain with a very long half-life. Studies suggest that men on average will accumulate 30 mg of cadmium in their bodies by the age of 502 . While the exact thresholds (ppm level) which cause harm are still contested, arsenic, lead and cadmium are known to affect human cardiovascular, neurological, renal, respiratory, gastrointestinal and reproductive functions3. Consequently, some regions are acting to lower cadmium limits in fertilizer and feed products due to these deleterious effects and the associated risks to human health.
Key technologies for metals removal
Researchers have developed several heavy metal removal technologies for the phosphates industry, with a particular focus on cadmium removal. Cadmium can also be dealt with at the mining and exploration stage.
Cadmium-rich phosphate resources can be specifically excluded from overall reserves estimates during exploration, for example, or reported separately as high cadmium/heavy metal content reserves. Selective mining of phosphate rock from multiple zones can then help avoid ore extraction from high cadmium parts of the deposit.
Cadmium can also be removed via thermal and chemical treatment of various products within the phosphate value chain. A large number of cadmium removal methods are described in the scientific literature. However, only a handful of these – calcination, co-crystallisation, ion exchange, precipitation and solvent extraction – are known to have been applied commercially at industrial-scale to date.
Key reasons behind the slow adoption of decadmiation techniques include:
• Lack of regulatory pressure
• Production of large hazardous waste streams requiring significant capital investments for additional treatment units Process flow sheet reconfiguration
• Waste management programmes with little or no economic benefit.
High-temperature calcination of phosphate rock (850-1150°C) can be used to volatilise elemental cadmium4. Cadmium removals in the range of 75-100 percent have been reported using this approach5. Calcination can be achieved at temperatures as low as 700°C in the presence of chloride salts, but may cause excessive corrosion of plant equipment6.
The only full-scale calcination plant for cadmium removal to date, at Nauru Phosphate Corporation7, was decommissioned in the late 1990s. The energy intensity of this process may partly explain its lack of adoption to date. Calcination can also require expensive exhaust scrubbing to prevent the atmospheric release of undesirable chemicals, particulates and radionuclides liberated by the process.
Impurities present as ions in phosphoric acid solution are amenable to elimination at high removal rates and efficiencies. This is therefore the basis of a large number of technology options for heavy metal removal from phosphate products.
In the mid-1990s, for example, Morocco’s phosphates research centre CERPHOS developed a co-crystallisation technique for removing heavy metals from phosphoric acid. This exploited the very high affinity of cadmium for anhydrite. The cadmium present in phosphoric acid will readily replace calcium in anhydrite crystals by isomorphic substitution due to their similar ionic radii8. The resulting cadmium-loaded anhydrite is then separated by filtration and discarded, similar to phosphogypsum.
Co-crystallisation can achieve cadmium removal in excess of 85% from feed levels of 170 mg Cd per kilo of phosphorus9. The technique does, however, have drawbacks. Suboptimal process conditions can inhibit crystal growth, for example, and result in poor acid recovery. The contaminated gypsum generated by the process is also unmarketable.
Cadmium and other metals can also be removed from phosphoric acid by precipitation as insoluble sulphides or metal-ligand complexes. These can then be filtered-out to obtain a cleaner phosphoric acid. Sodium, hydrogen or organic sulphides all make suitable anion sources (S2-). These are injected into or pumped through the acid solution – in the form of organothiophosphates – to initiate precipitation.
Elements such as copper, zinc, and iron are also precipitated. This makes the process less selective for cadmium and generates large volumes of toxic sludge. The precipitation process may also release highly toxic hydrogen sulphide gas, depending on the reagent used. This increases overall treatment costs and risks exposure to hazardous chemicals.
Adsorption – the capture of metal ions using adsorbents – is another technology for removing cadmium from solutions of phosphoric acid. Suitable adsorbents include synthetic materials, naturally occurring substances, mining overburden, and materials based on micro-organisms, as well as agricultural and agro-industrial wastes10. The successful removal of heavy metals from acid mine waters are reported by numerous adsorption research studies, suggesting that adsorbents could be successfully applied as a process solution for phosphoric acid.
“Researchers have developed several heavy metal removal technologies for the phosphates industry, with a particular focus on cadmium removal.
Cementation offers a similar approach. In this process, metal ions are precipitated at the solid interface of a sacrificial metal, sometimes in conjunction with an organic reagent. While cadmium removal rates from wastewater of up to 95% have been successfully reported by researchers11, scientific studies for phosphoric acid are more limited. Pilot-scale and plant-scale cementation testing, however, has yielded phosphoric acid containing less than 1 ppm and 8 ppm cadmium, respectively12.
In solvent extraction, selective mass transfer of cadmium and other metals takes place from aqueous phosphoric acid to an organic solvent. The metal loaded organic solvent is then separated and regenerated for re-use after being stripped of cadmium. Solvent extraction is currently a prevalent technology for extracting copper, nickel, uranium and cobalt from sulphuric acid leach solutions.
Membrane extraction is a similar technology. In this high selectivity technique, mass transfer from the source phase to the receiver phase takes place across supported liquid or solid film membranes – the key drivers being electrochemical potential, pH, hydrostatic pressure or concentration gradient across the two phases.
Information on the large scale viability of these extraction methods is limited, as most of the research work has been carried out at lab scale. Nonetheless, solvent extraction has been demonstrated at industrial scale (30,000 t/a capacity) at a US phosphoric acid purification plant. The licensor, KEMWorks, reported a successful reduction in cadmium from 125 mg/L to less than 5 mg/L, with low capital and operating costs13.
In general, solvent extraction processes require pre- and post-treatment of phosphoric acid for effective separation and to remove residual solvents, especially when producing food-grade phosphoric acid or phosphates. The large volumes of solvent needed also add to process costs4 and are another potential drawback.
The efficacy of ion exchange for heavy metals removal, using both anion and cation exchange resins, has been investigated. Research has, however, mostly focused on water and wastewater treatment, with only limited studies of phosphoric acid applications.
Anion exchange resins show a greater selectivity for cadmium, versus cation exchange resins, and are therefore the preferred media for removal from phosphoric acid. The cadmium ions present need to be converted into anionic complexes as a pre-treatment step (generally with halides in a reducing environment) before being selectively removed by the anionic exchange media.
In the mid 1990s, anion exchange was successfully tested at pilot-scale for cadmium removal from phosphoric acid in combination with the hemihydrate-dihydrate (HDH) process – with some researchers even recommending this as a Best Available Technology (BAT)14. Cadmium was reduced to less than 2 mg of Cd per kg of P2O5 with treatment costs as low as $15-25 /t P2O515.
Nonetheless, ion exchange technology has significant drawbacks. These include poor selectivity (especially against divalent ions), the need to pre-treat the phosphoric acid (clarification to prevent media bed clogging), equipment corrosion (from chloride ions) and the careful handling and disposal of the resin regeneration solutions.
ACCO-PHOS® : Syensqo’s heavy metal removal solution
From the above literature review, it is clear that several technological options for effective heavy metals removal from phosphate rock or phosphoric acid – especially for cadmium – are currently available to producers. However, these options all suffer from various downsides, including high ‘in-use’ reagent costs or high capital intensity.
Syensqo has developed ACCO-PHOS® technology to specifically address the increased demand for heavy metal removal within the phosphate industry. This family of reagents avoids many of the usual pitfalls and downsides – thereby offering a more attractive and effective commercial option for the removal of heavy metals from weak and strong phosphoric acid. Critically, ACCO-PHOS® reagents are particularly selective for cadmium and arsenic.
ACCO-PHOS® TECHNOLOGY: MAIN FEATURES AND BENEFITS
Bolt-on technology
• No need for heavy capital expense
• Additional unit operations/processes unnecessary
• Add it neat to the acid at the recommended point
• Dosage directly proportional to the concentration of metal impurities
High heavy metal removal efficiency
• One reagent product is suitable for multiple metals (Cd, As, Cu, Pb, Hg) depending on the concentration of competing ions
• Complementary solution available in higher dosage situations because of competing metal ion concentrations
Customised to process conditions
• Can work at different residence times – from a few seconds to several hours Can work at range of temperatures – from room temperature to 80°C
• Stable heavy metal-ACCO-PHOS® complex formed
Application flexibility
• Weak acid slurry (reactor slurry to filters)
• Strong acid (merchant grade)
• Super phosphoric acid
Proven technology
• Highly efficient and consistent performance in numerous lab tests and plant trials
• Robust to diverse ore and plant conditions
• Supported by Syensqo’s strong R&I capabilities and deep application expertise in servicing global mining industries for over a century
Using proprietary chemistry, ACCO-PHOS® forms an insoluble complex with heavy metals in the acidic solution. Advantageously, the reagents can be applied at multiple points of the phosphoric acid production and purification process, as shown in Figure 1.
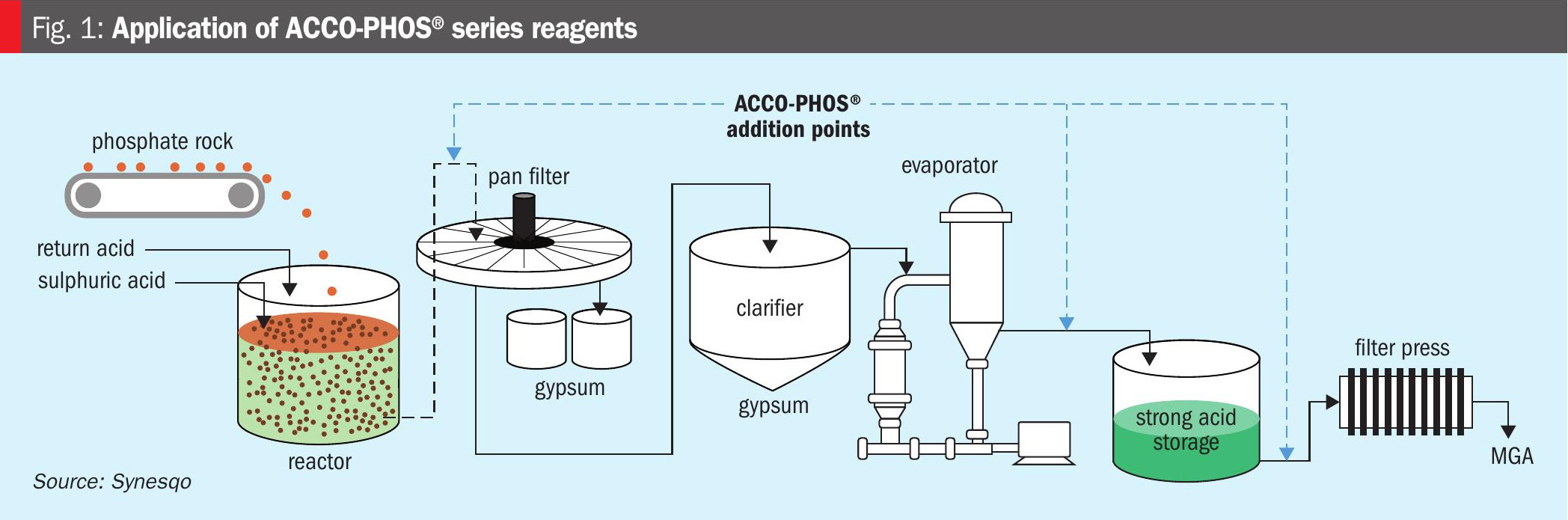
ACCO-PHOS® is a ‘bolt-on’ technology that requires only minimal capital investment for most applications. The need to separate the precipitated heavy metal solids formed from phosphoric acid streams is generally the only additional process requirement. These precipitates adsorb onto the surface of solids within the system, such as phosphogypsum, and can therefore be filtered out. The ACCO-PHOS®-heavy metal complex that forms is also less dense than concentrated acid, allowing for removal via gravity separation or centrifugation.
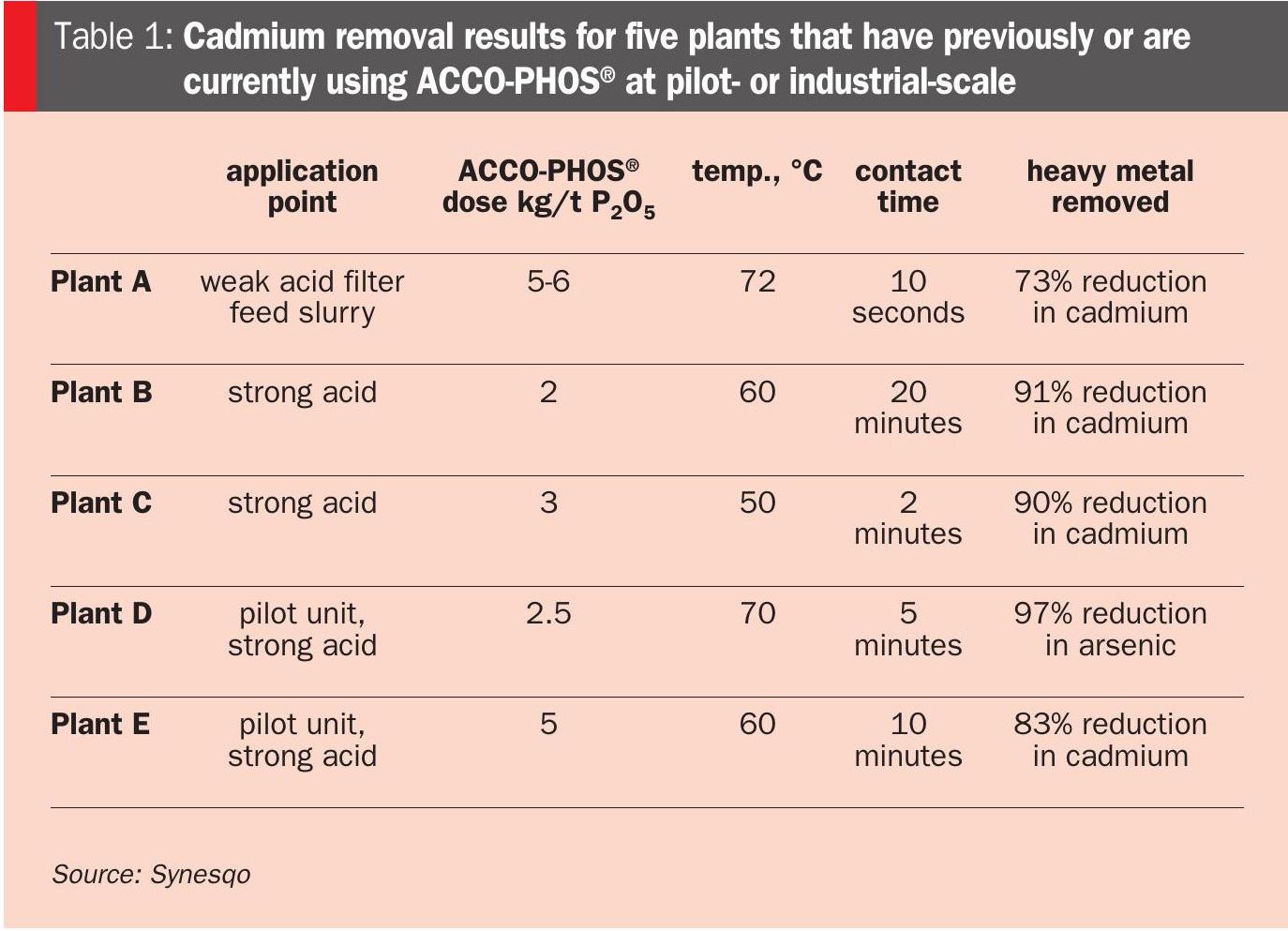
ACCO-PHOS® technology has been proven at an industrial scale, with multiple plants consistently purchasing commercial quantities to purify phosphoric acid. Table 1 provides a list of plants that have used, or are currently using, ACCO-PHOS® at pilot- or industrial-scale, together with pertinent application details and performance results for cadmium removal.
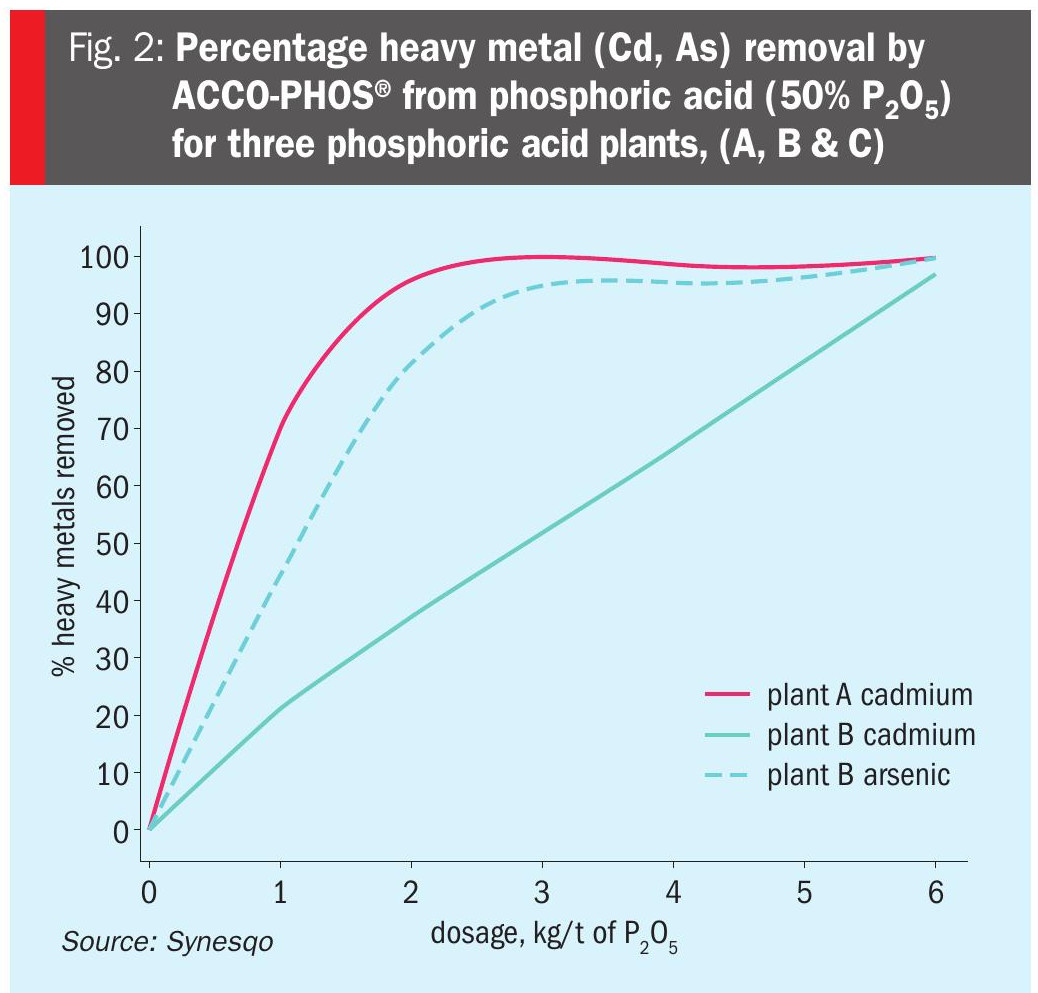
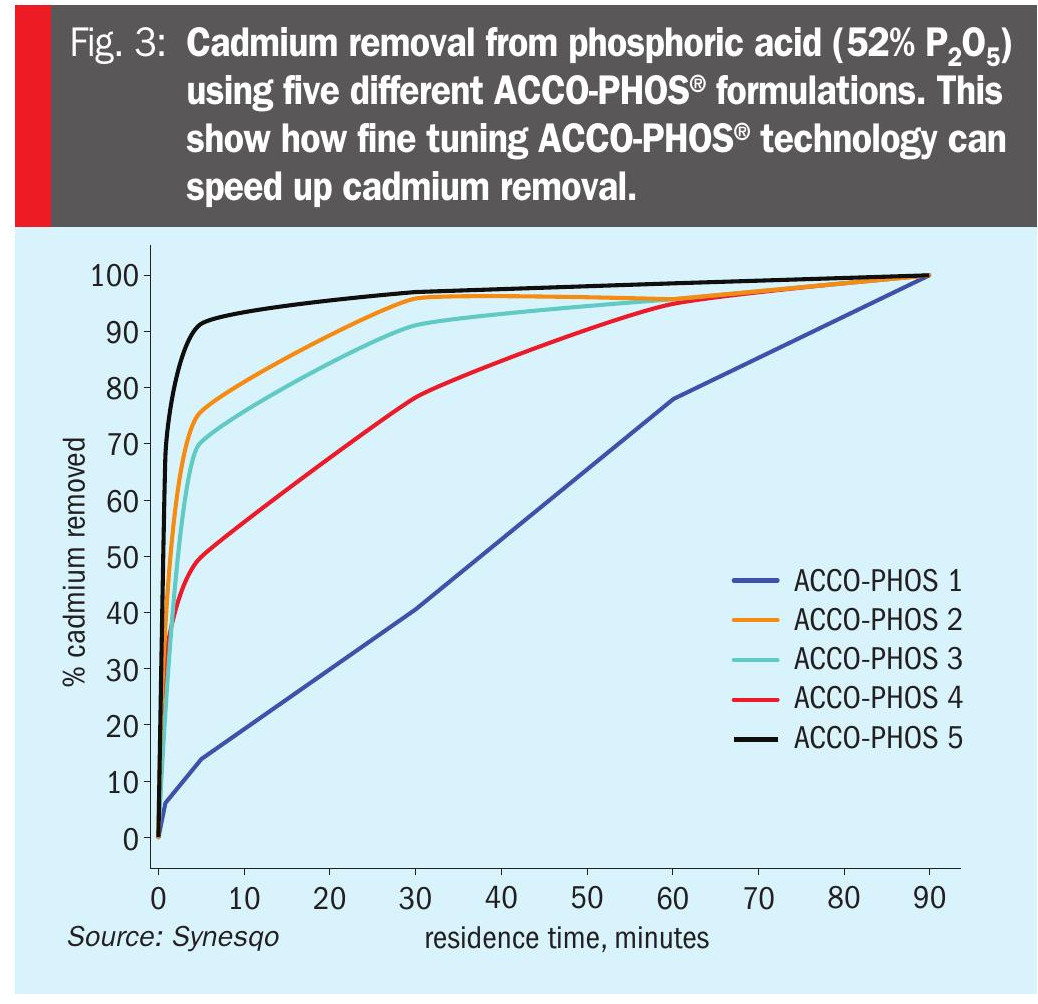
Two charts are included to illustrate the efficacy of ACCO-PHOS® technology for the commercial removal of heavy metals at phosphoric acid plants. Figure 2 shows the percentage heavy metal (Cd, As) removal by ACCO-PHOS® from phosphoric acid (50% P2 O5) for three phosphoric acid plants, (A, B & C). Figure 3, meanwhile, shows the percentage cadmium removal from phosphoric acid (52% P2 O5) using five different ACCO-PHOS® formulations. This illustrates how fine tuning ACCO-PHOS® technology can speed up cadmium removal by altering reaction kinetics and/or dispersion.
ACCO-PHOS® is a robust ‘bolt-on’ technology that requires minimal capital outlay. The reagent can be applied to reduce the cadmium and arsenic content of the treated acid to the desired level in one of two ways (Figure 1):
• As a single-stage addition to concentrated acid
• By multi-stage additions to weak or concentrated acid.
The extent of heavy metal removal and dosing requirements – being influenced by the concentration of other competing ions such as zinc, copper and mercury – will vary according to the chemical characteristics of the phosphoric acid.
Summary
As discussed in this article, while a number of decadmiation technologies have been demonstrated at the laboratory- or pilot-scale, only a select few have gone on to be implemented at industrial-scale. The addition of process chemicals required by many of these technologies can be deleterious, having a negative impact on product quality or process efficiency. Examples include the formation of large volumes of toxic sludge, spent adsorbent or solvent, all requiring costly disposal and careful waste management planning. These drawbacks have generally discouraged fertilizer producers from adopting these technologies at industrial-scale.
In contrast, Syensqo’s ACCO-PHOS® reagents provide a simple, effective and economical method of reducing cadmium and arsenic levels in phosphoric acid. Importantly, it is a ‘future proof’ technology that can meet both current and potential future regulatory requirements. Beneficially, these reagents can also provide phosphate producers with the flexibility to develop and produce new low heavy metal content fertilizers, feed phosphates and purified phosphoric acid.
ACCO-PHOS® reagents are already being actively adopted by fertilizer producers at industrial scale. And this is just the start. Looking ahead, Syensqo is committed to investing in the development of more selective, efficient and sustainable technologies to remove other heavy metals of concern.
References